An accurate and low cost paper-based strip test for Covid-19 has been approved for commercial launch by the Drugs Controller General of India.
Indian scientists have come up with a new testing method called Feluda, a test which is similar to taking samples through a PCR swab test but is more reliable and simpler to use.
It will cost 500 Indian rupees – about 6 euros. Kits are expected to reach the market shortly.
The test was named after a famous fictional Bengali detective, though its full name is: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) Feluda test.
The development comes as India is conducting an average of 1 million tests daily, with the nationwide caseload surpassing 6.7 million – the world's second-highest behind the United Sates. More than 100,000 people in the country have died of the coronavirus so far.
Feluda was developed by a research team led by Debojyoti Chakraborty and Souvik Maiti of the Council of Scientific and Industrial Research (CSIR) and leading Indian conglomerate, the Tata Group.
The test matches the accuracy level of RT-PCR tests, considered the gold standard in diagnosis of Covid-19, and has a quicker turnaround time and requires less expensive equipment.
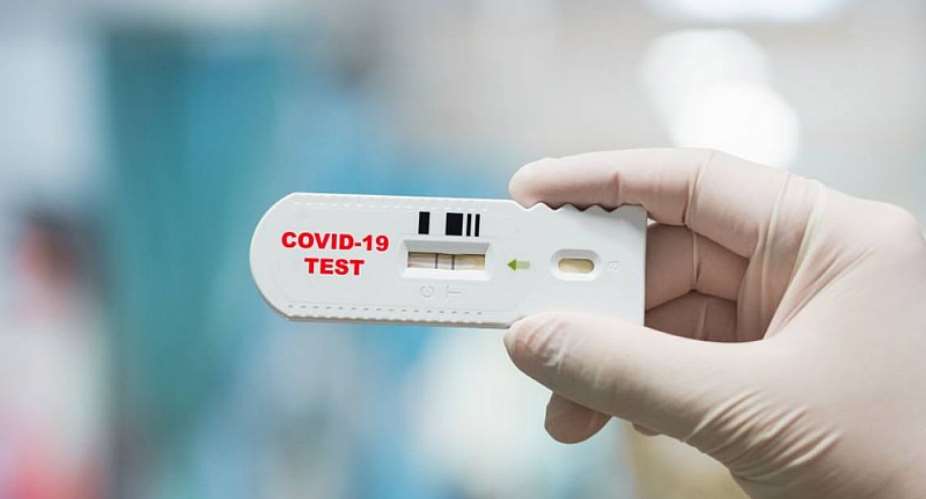
Similar to a pregnancy strip test, Feluda changes colour if the virus is detected and doesn't need expensive machines for detection. Two blue lines indicate a positive result, while a single blue line means the test has returned negative.
"The CRISPR technology uses a highly specific CAS9 protein to find and bind to the target Covid signature. This is then coupled with paper-strip chemistry to elicit a visual readout on a paper strip," said Chakraborty.
The tests are so specific that they can distinguish SARS-CoV-2 infections from other coronaviruses such as the one that caused the SARS pandemic in 2002-03.
“The Tata CRISPR test has met high quality benchmarks, with 96 percent sensitivity and 98 percent specificity for detecting novel coronavirus,” a CSIR statement said.
Based on a gene-editing technology, it finds its use in correcting genetic defects and treating and preventing the spread of diseases. The CRISPR technology can detect specific sequences of DNA within a gene and uses an enzyme functioning as molecular scissors to snip it.
“It's a very accurate and accepted testing method and the advantage is that it can be used in any lab that has the technology,” said Dr Nirmal K Ganguly, former director-general of the Indian Council of Medical Research.
India is currently conducting Covid-19 testing through the real-time polymerase chain reaction (RT-PCR) test and the rapid antigen test (RAT). While an RT-PCR test gives a result in 1.5 hours, a rapid antigen test takes 30 minutes.
In a related development, India's drug regulator has knocked back a proposal from Dr Reddy's Laboratories Ltd to conduct a large study in the country to evaluate Russia's Sputnik-V Covid-19 vaccine and has asked it to first test the vaccine in a smaller trial.
After Russia became the first country that claimed to have come up with an effective Covid-19 vaccine, Dr Reddy's Lab joined hands with the Russian Direct Investment Fund (RDIF) to conduct clinical trials of the Sputnik V vaccine as well as its distribution.
Experts, however, raised concerns over the safety of the vaccine as it was rolled out in such a short time.




 Elisu By-election: "If you call yourself a man, boo Chairman Wontumi again" — Bo...
Elisu By-election: "If you call yourself a man, boo Chairman Wontumi again" — Bo...
 Fuel tanker driver escapes with his life after tanker goes up in flames near Suh...
Fuel tanker driver escapes with his life after tanker goes up in flames near Suh...
 Uniform change: ‘Blue and white are brighter colours’ — Kwasi Kwarteng explains ...
Uniform change: ‘Blue and white are brighter colours’ — Kwasi Kwarteng explains ...
 MoE not changing all public basic school uniforms but only newly built ones — Kw...
MoE not changing all public basic school uniforms but only newly built ones — Kw...
 We’re only painting new public basic schools blue and white – Dr. Adutwum clarif...
We’re only painting new public basic schools blue and white – Dr. Adutwum clarif...
 Bawumia has lost confidence in his own govt’s economic credentials – Beatrice An...
Bawumia has lost confidence in his own govt’s economic credentials – Beatrice An...
 I fought WW2 at age 16 – WO1 Hammond shares At Memoir Launch
I fought WW2 at age 16 – WO1 Hammond shares At Memoir Launch
 GRA-SML deal: Regardless of what benefits have been accrued, the contract was aw...
GRA-SML deal: Regardless of what benefits have been accrued, the contract was aw...
 April 26: Cedi sells at GHS13.75 to $1, GHS13.18 on BoG interbank
April 26: Cedi sells at GHS13.75 to $1, GHS13.18 on BoG interbank
 Champion, promote the interest of women if you become Vice President – Prof. Gya...
Champion, promote the interest of women if you become Vice President – Prof. Gya...
