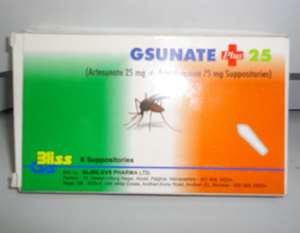
The Food and Drugs Authority (FDA) has re-emphasized its call to the general public to avoid patronizing an anti-malaria medicine for children called Gsunate Plus.
According to the authority, no clinical trial study had been conducted on the product made up of the combination of Artesunate 25mg and Amodiaquine 75mg.
Investigations conducted by the FDA revealed that Gsunate Plus, manufactured in India by BLISS GVS PHARMA LTD, located at 10 Dewan Udyog Nagar, Aliyali Palghar Maharashtra- 401, 404 India, had neither been registered nor approved by the Medicine Regulatory Authority in India for sale or use.
'The efficacy of the combination of artesunate and amodiaquine through the rectal route has not been established and therefore treatment of malaria in children with this drug could lead to therapeutic failures with complications,' Dr Stephen K. Opuni, Chief Executive of FDA said.
He said the Gsunate Plus suppository had not been registered by the FDA and therefore the drug cannot be guaranteed for the treatment of malaria in children.
'Hospitals, clinics, pharmacies, licensed chemical sellers and other health facilities having stocks of Gsunate Plus Suppository are reminded to immediately stop dispensing them and handover stocks to the nearest FDA office countrywide for safe disposal,' he said.
Meanwhile, the importer, Tobinco Pharmaceutical Ltd, is still assisting the Food and Drugs Authority (FDA) to ensure a total recall of the drug from the market.
By Jamila Akweley Okertchiri




 Minority will expose the beneficial owners of SML, recover funds paid to company...
Minority will expose the beneficial owners of SML, recover funds paid to company...
 Prof. Opoku-Agyemang has ‘decapitated’ the NPP’s strategies; don’t take them ser...
Prof. Opoku-Agyemang has ‘decapitated’ the NPP’s strategies; don’t take them ser...
 Abubakar Tahiru: Ghanaian environmental activist sets world record by hugging 1,...
Abubakar Tahiru: Ghanaian environmental activist sets world record by hugging 1,...
 Prof. Naana Opoku-Agyemang will serve you with dignity, courage, and integrity a...
Prof. Naana Opoku-Agyemang will serve you with dignity, courage, and integrity a...
 Rectify salary anomalies to reduce tension and possible strike action in public ...
Rectify salary anomalies to reduce tension and possible strike action in public ...
 Stop all projects and fix ‘dumsor’ — Professor Charles Marfo to Akufo-Addo
Stop all projects and fix ‘dumsor’ — Professor Charles Marfo to Akufo-Addo
 Blue and white painted schools will attract dirt shortly – Kofi Asare
Blue and white painted schools will attract dirt shortly – Kofi Asare
 I endorse cost-sharing for free SHS, we should prioritise to know who can pay - ...
I endorse cost-sharing for free SHS, we should prioritise to know who can pay - ...
 See the four arsonists who petrol-bombed Labone-based CMG
See the four arsonists who petrol-bombed Labone-based CMG
 Mahama coming back because Akufo-Addo has failed, he hasn't performed more than ...
Mahama coming back because Akufo-Addo has failed, he hasn't performed more than ...
