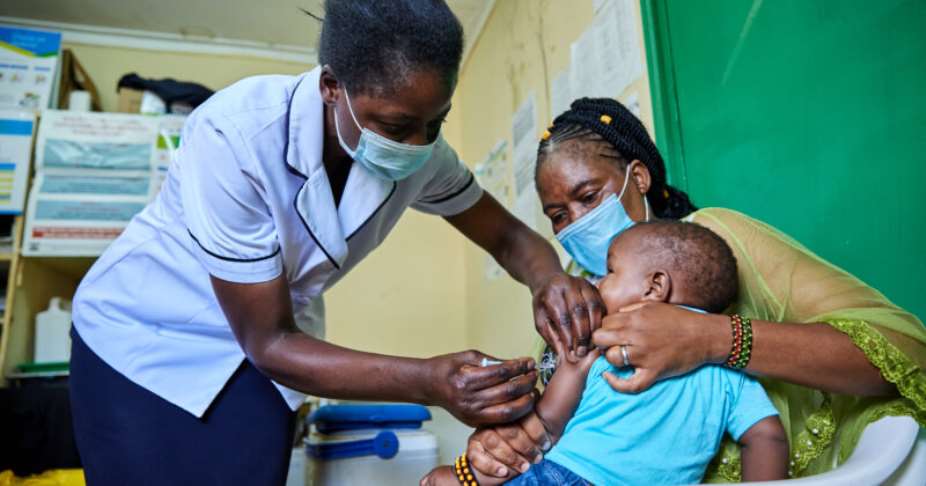
In April 2023, almost coinciding with World Malaria Day, the West African nation Ghana approved the malaria vaccine R21, even before the WHO approved it. The vaccine R21 is developed at the University of Oxford and produced by the world’s largest vaccine manufacturer, Serum Institute of India. The Ghana Food and Drugs Authority (FDA) approved the vaccine for children between five and thirty-six months, the age group at the highest risk of death from malaria. And on 2nd October 2023, the World Health Organization (WHO) approved a new vaccine, R21/Matrix-M, to prevent malaria in children. And this vaccine is produced and scaled up by the Indian vaccine manufacturer Serum Institute of India (SII).
Clinical trials for this specific vaccine have been conducted in the UK, Thailand, and various African nations. This includes a Phase III experiment with 4,800 children in Burkina Faso, Kenya, Mali, and Tanzania. Earlier, the 2021 Phase IIb trial data demonstrated 77% effectiveness and a comforting safety profile after administering three primary doses and a one-year booster. In August, Burkina Faso became the 3rd African country to approve the vaccine before the formal approval from the WHO. Now, the WHO has approved it for usage, as during the first two phases, the vaccine achieved the WHO-specified 75% effectiveness threshold.
Before the R21/Matrix-M vaccine, GlaxoSmithKline (GSK) plc developed and manufactured the first malaria vaccine, Mosquirix, also known as RTS, S/AS01. The project was financially supported by The Bill and Melinda Gates Foundation (BMGF) under the PATH Malaria Vaccine Initiative (MVI). Since 2019, pilot programmes introducing the RTS, S vaccine have been rolled out in Ghana, Kenya and Malawi, and more than 1.7 million children have already benefitted from at least one dose of Mosquirix.
Twelve nations in various parts of Africa, including Ghana, Kenya, and Malawi, have already been in agreement and are anticipated to receive 18 million doses of the RTS, S malaria vaccine over the following two years. Nine more countries have raised their demand to receive the vaccine. In response to the growing demand, in 2021, GSK entered a product transfer partnership with Indian vaccine manufacturer Bharat Biotech. However, the first vaccine doses of the RTS, S should arrive in these countries in the final quarter of 2023 and begin to be administered by early 2024. On the other hand, the R21 vaccine will be available for use only after mid-2024.
Both vaccines have been adequately tested and are safe. They have not been put to a side-by-side trial, and there is no direct competition between R21 and RTS, S. Further, there is no evidence so far that one vaccine works more effectively than the other. As per WHO, both vaccines have a similar efficacy rate when given under the same circumstances. However, according to researchers, R21 will be easier to produce and at a lower cost per dose. In any case, a country would be free to choose one of the vaccines based on its programmatic factors, availability, and affordability.
Over 600,000 people die yearly from malaria, primarily newborns and children from Africa. Due to several complexities and a lack of technologies, developing an affordable vaccine against the disease has taken decades. The WHO approved the first malaria vaccine, GSK’s Mosquirix, only two years ago. Unfortunately, the demand still outnumbers the current production capacity. Therefore, the approval of the second vaccine represents a crucial advancement in the fight against malaria, particularly for Africa.
Indeed, malaria remains the most significant cause of death among children in Africa. In 2021, Africa accounted for almost 95% of malaria cases and 96% of malaria deaths worldwide. Four African countries- Nigeria, the Democratic Republic of the Congo (DRC), Tanzania, and Niger accounted for more than half of global deaths that occurred due to malaria.
By 2026 alone, the demand for malaria vaccines is projected to reach 40 to 60 million doses annually. Further, it may cross 80 to 100 million annually by 2030. Projecting ahead, Serum Institute is prepared to produce 100 million doses per annum immediately and is planning to produce twice as much over the next two years.
Today, India is a global powerhouse for pharmaceutical manufacturing. Therefore, India’s experience, manufacturing excellence, and cost-effectiveness are essential for assuring the successful production and marketing of R21. Not only the accessibility of vaccines, but their affordability, particularly in developing nations, is also a crucial factor in the fight against disease. In this sense, Serum Institute has taken a highly significant public pledge to maintain the price of the vaccines at $3 or less. Moreover, the vaccine has a storage life of 24 months if stored in a refrigerator at 2 to 8 degrees Celsius. This is an achievable threshold for developing countries compared to many other vaccines requiring much colder storage.
Whether it is Bharat Biotech or Serum Institute of India, the success of the global distribution of malaria vaccine will depend significantly on India’s vaccine manufacturing prowess. India currently contributes to around 60% of global vaccine production and 90% of the measles vaccines globally. As a matter of fact, 65-70% of WHO measles vaccines are procured from India. During the COVID-19 outbreak, about 101 countries and two UN entities received more than 282 million vaccine doses of vaccines to COVID-19 vaccines from India.
Going forward, Serum Institute is exploring the possibilities of locally manufacturing the vaccines in Ghana. Local firm DEK Vaccines Ltd., collaborating with the Serum Institute, intends to set up a factory in Ghana’s capital, Accra. They are collaborating to figure out how to produce and finish R21 in Ghana. As of now, Ghana does not make its vaccines. DEK Vaccines Limited, however, is confident and anticipates beginning local production within the next two years. Similarly, Biovaccines Nigeria Limited has signed a contract with Serum Institute for local production. The company is a joint venture between the Government of Nigeria and May & Baker Nigeria Plc. Clearly, this new vaccine represents a giant step for India’s vaccine diplomacy with Africa.




 Pointing out the ills of NPP government not enough; tell us what you can do — Mi...
Pointing out the ills of NPP government not enough; tell us what you can do — Mi...
 April 27: Cedi sells at GHS13.78 to $1, GHS13.18 on BoG interbank
April 27: Cedi sells at GHS13.78 to $1, GHS13.18 on BoG interbank
 Worry about the furniture problem in basic schools; not how to paint schools in ...
Worry about the furniture problem in basic schools; not how to paint schools in ...
 Ramaphosa lauds ANC record as S.Africa celebrates democracy
Ramaphosa lauds ANC record as S.Africa celebrates democracy
 Ghana needs new counter-terrorism Act to tackle radicalised youth – CISA
Ghana needs new counter-terrorism Act to tackle radicalised youth – CISA
 Kumasi Central Prison triple its capacity to 1800 inmates
Kumasi Central Prison triple its capacity to 1800 inmates
 By-election: Ejisu is destined for NPP – Ahiagbah
By-election: Ejisu is destined for NPP – Ahiagbah
 Don't travel to volatile regions in northern Mali — Ministry of Foreign Affair t...
Don't travel to volatile regions in northern Mali — Ministry of Foreign Affair t...
 ILO’s claim of depleting reserves false, we have funds to pay pensions — SSNIT
ILO’s claim of depleting reserves false, we have funds to pay pensions — SSNIT
 Benin police fire tear gas to break up union protest
Benin police fire tear gas to break up union protest
