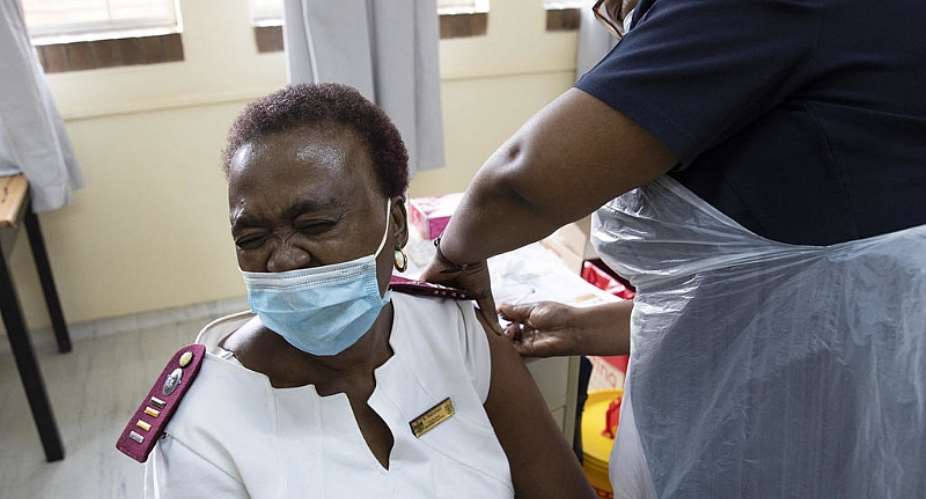
Authorities in South Africa should lift a suspension of use of the Johnson & Johnson Covid-19 vaccine if a number of conditions are met, the country's health regulator has announced.
South African Health Products Regulatory Authority (SAHPRA) said it had “recommended that the pause in the Sisonke study be lifted, provided that specific conditions are met”, referring to the clinical trial that allows Pretoria to make the single dose J&J vaccine available to healthcare workers.
The conditions laid out by SAHPRA include screening and monitoring of those at high risk of blood clotting disorders, as well as precautions to manage any cases of thrombosis or thrombocytopenia that arise from participants in the trial who develop clotting.
Thrombosis happens when a blood clot forms inside a blood vessel, while thrombocytopenia describes a low platelet count, when your blood contains less of the cells needed to help clot blood.
South Africa's government had suspended the rollout of the J&J vaccine on Tuesday, following a move by US health authorities to pause its use after identifying rare blood clots in six people, out of a total of about 7 million people who already had the jab in the US.
The Sisonke study involves the rollout of the viral vector J&J vaccine while the licensing process still takes place, although the South African Medical Research Council (SAMRC) has said that despite the vaccine not yet being licensed, this does not mean it is not safe and effective.
“Participants in the Sisonke study will be informed about the possible risks of developing a blood clotting disorder after vaccination,” said SAHPRA. “They will also be advised to seek immediate medical assistance if they develop early signs and symptoms associated with blood clots or low platelet counts.”
Importance of J&J vaccine
The J&J vaccine has become a vital tool in the fight against Covid-19 in South Africa, given that the authorities decided that the AstraZeneca was not as effective against a local variant of the coronavirus.
Although this decision has drawn the irk of some South African experts who questioned whether the government really determined the AstraZeneca vaccine's effectiveness, as well as Pretoria's decision to send it to other African countries.
The J&J vaccine is also going to play a key role in helping to vaccinate the entire African continent, as South African President Cyril Ramaphosa helped broker a deal to secure J&J doses manufactured locally by Aspen Pharmacare that will be made available through the African Vaccine Acquisition Task Team (AVATT).
Rare cases of blood clotting disorders following Covid-19 vaccinations originally surfaced in relation to the AstraZeneca vaccines, prompting a number of countries to suspend their use and some countries, such as Denmark, banning their use entirely.
Case for blood clotting overstated?
The science behind such suspensions of AstraZeneca or J&J vaccines still remains unclear, with recent studies, such as one published by Oxford University reporting that the risk of rare blood clotting is much higher when contracting Covid-19 rather for vaccination itself.
The Oxford University research also compared the risk of thrombosis with the AstraZeneca vaccine to other vaccines developed using new mRNA technology, such as Pfizer or Moderna.
Cases of rare clotting called Cerebral Venous Thrombosis (CVT) amongst those receiving a mRNA vaccine stood at 4 people in one million and in those receiving an AstraZeneca vaccine at 5 in one million.
This perhaps suggests that the risk of blood clotting from either viral vector vaccines or mRNA vaccines was statistically the same.
South Africa is one of the hardest hit by Covid-19 in sub-Saharan Africa with 1.56 million cases of the coronavirus, and more than 53,660 deaths, according to the Africa Centres for Disease Control and Prevention.
The South Africa regulator said resumption of the J&J rollout would also need the approval of the relevant medical ethics research committees.
Pretoria has ordered some 30 million doses of the J&J vaccine, according to the Associated Press, who reported that South Africa had vaccinated 290,00 health care workers, all with the J&J vaccine, out of a population of 60 million people.




 SSNIT must be managed without gov’t interference – Austin Gamey
SSNIT must be managed without gov’t interference – Austin Gamey
 Ejisu by-election could go either way between NPP and independent candidate — Gl...
Ejisu by-election could go either way between NPP and independent candidate — Gl...
 We never asked ministers, DCEs to bring NPP apparatchiks for returning officer r...
We never asked ministers, DCEs to bring NPP apparatchiks for returning officer r...
 No one denigrated the commission when you appointed NDC sympathizers during your...
No one denigrated the commission when you appointed NDC sympathizers during your...
 Used cloth dealers protests over delayed Kumasi Central Market project
Used cloth dealers protests over delayed Kumasi Central Market project
 A/R: Kwadaso onion market traders refuse to relocate to new site
A/R: Kwadaso onion market traders refuse to relocate to new site
 Dumsor: Corn mill operators at Kaneshie market face financial crisis
Dumsor: Corn mill operators at Kaneshie market face financial crisis
 Jamestown fishermen seek support over destruction of canoes by Tuesday's heavy d...
Jamestown fishermen seek support over destruction of canoes by Tuesday's heavy d...
 Election 2024: EC to commence voter registration exercise on May 7
Election 2024: EC to commence voter registration exercise on May 7
 Western Region: GWL hopeful of restoration of water today in Sekondi-Takoradi
Western Region: GWL hopeful of restoration of water today in Sekondi-Takoradi
