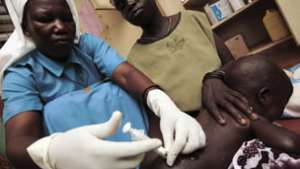
When it comes to immunization, we in Ghana are used to taking the lead. We have stood out among our neighbours by maintaining high childhood vaccination coverage, while also leading the way with successful new vaccine introductions.
Exactly seven years ago, Ghana introduced pneumococcal and rotavirus vaccines, which marked the first simultaneous introduction of two new vaccines for an African country. This year, we are again leading the way by introducing the first malaria vaccine in selected parts of the country.
This an important milestone. While we have made progress in reducing the malaria burden on families and communities, the disease is still rampant in Ghana, and it takes its toll particularly on young children. By implementing this vaccine in a few areas of the country at first, health officials can gather data and information necessary to decide how a malaria vaccine could be added to the broader arsenal of malaria tools and strategies currently in use.
This is also an important milestone because we place such high value on homegrown solutions for health. Much of the important data we use to determine the introduction of vaccines and other health interventions tend to come from overseas. In this phased introduction, the data we generate in Brong Ahafo, Central, and Volta Regions will help the country decide on the wider use of a malaria vaccine in Ghana and potentially in other areas in Africa where malaria poses a public health problem.
If the phased introduction goes as planned, we should have local data and information to determine—after vaccinating roughly 200,000 children from around 6 months to 24 months of age—how best to deliver four doses of the vaccine, the potential of the vaccine to reduce deaths among children, and its safety profile when used routinely.
Ghana has experience with this malaria vaccine. When the vaccine was tested across seven countries in a five-year clinical trial that ended in 2014, more than 2,500 Ghanaian children took part at sites in Agogo and Kintampo. Our own scientists participated in the vaccine’s development. They came from a range of institutions, including the School of Medical Sciences at the Kwame Nkrumah University of Science and Technology; Agogo Presbyterian Hospital; and the Ghana Health Service’s Kintampo Health Research Centre.
Following a review of the data from that clinical trial, global authorities decided to move the vaccine into the phased introduction. Based on the data, a stringent European regulatory authority, the European Medicines Agency, issued a positive scientific opinion of the vaccine.
The World Health Organization (WHO) then adopted the advice of its global advisory committees for malaria and immunization and recommended the introduction of the vaccine in selected parts of Africa. Ghanaian regulatory authorities have also reviewed the data and cleared the vaccine for use in this phased introduction.
In Ghana, this step to introduce the vaccine in selected areas builds on our experience with the clinical trial. When WHO issued a call for African countries to express interest in the phased introduction, we were one of 10 countries that responded positively. Our selection, along with Kenya and Malawi, meant that we could implement the only vaccine that has shown a protective effect against malaria among young children in a Phase 3 trial. We were selected, in part, because of our high-performing malaria and immunization programmes. We elected to participate because we know how to deliver vaccines. We also know that immunization is one of the most cost-effective ways to address some of the worst health problems in our communities, measles being a typical example.
Kwadwo Odei Antwi-Agyei managed the Expanded Programme on Immunisation in Ghana from 2003 to 2013.




 Avoid pre-registered SIMs, buyer and seller liable for prosecution – Ursula Owus...
Avoid pre-registered SIMs, buyer and seller liable for prosecution – Ursula Owus...
 Election 2024: Mahama has nothing new to offer Ghanaians, Bawumia is the future ...
Election 2024: Mahama has nothing new to offer Ghanaians, Bawumia is the future ...
 OSP files fresh charges against ex- PPA Boss
OSP files fresh charges against ex- PPA Boss
 Withdraw unreasonable GH¢5.8m fine against former board members – ECG tells PURC
Withdraw unreasonable GH¢5.8m fine against former board members – ECG tells PURC
 Akroma mine attack: Over 20 armed robbers injure workers, steal gold at Esaase
Akroma mine attack: Over 20 armed robbers injure workers, steal gold at Esaase
 Those who understand me have embraced hope for the future — Cheddar
Those who understand me have embraced hope for the future — Cheddar
 Ghana will make maiden voyage into space should Bawumia become President — Chair...
Ghana will make maiden voyage into space should Bawumia become President — Chair...
 Train crash: Despite the sabotage, we shall not be deterred and will persevere —...
Train crash: Despite the sabotage, we shall not be deterred and will persevere —...
 Tema-Mpakadan railway project a perversion of the original viable concept design...
Tema-Mpakadan railway project a perversion of the original viable concept design...
 Train crash: Elsewhere, everyone involved in the test will either be fired or re...
Train crash: Elsewhere, everyone involved in the test will either be fired or re...
