Almost every country is affected negatively by the COVID-19 pandemic. As of mid-March 2022, approximately 470 million cases and 6 million deaths had been reported worldwide to the World Health Organization (WHO) since the outbreak. Despite the rapid development of effective and safe COVID-19 vaccines, new variants of the virus that are highly transmissible and can evade the effectiveness of available vaccines are emerging. Even though over 10 billion doses of COVID-19 vaccines have already been administered, the world is still unable to end the pandemic due to the vaccine's uneven distribution. Many countries still lag behind the required vaccine doses to achieve herd immunity.
According to a statement issued by the Multilateral Leaders Task Force at its 8th meeting in early March 2022, 23 countries were yet to fully vaccinate 10% of their populations, 73 countries were yet to achieve 40% coverage, and many more were expected to fall short of the 70% target by the middle of this year. This pandemic threat is continuously instigating scientists, researchers, and funding collaborators into developing varied but innovative and efficacious COVID-19 vaccines that are both affordable and can be locally produced, especially in developing countries, to ensure their sustainable production, distribution, and administration. One of these emerging technologies is the egg-based vaccine development, which is undergoing verification, and was inspired by the techniques, materials, and technology used to develop influenza and yellow fever vaccines.
Vaccines are made up of some components of a disease-causing pathogen to increase immunity to prevent or treat the disease. They can be made in several ways. From a living organism that has been weakened by genetic modification to reduce its ability to cause disease; from a whole organism that has been inactivated by chemical, thermal or other means; from disease-causing organism components such as specific proteins or nucleic acids; and from the linkage of polysaccharides to proteins. In some cases, scientists combine different vaccines to protect against multiple diseases.
Dr. Eluemuno Blyden, CEO of Avril Biopharma Incorporated, and the Mother of Birds Initiative stated in the third edition of The COVID-19 Diary's webinar run by the Association of African Universities TV (AAU TV) under the theme: “Vaccine Development, Variants, Herd Immunity” that understanding the genetics of a changing virus and how it evolves in real-time and the genetics of a nation's population, are required in a pandemic to identify a pathogen, qualify suitable targets in its life cycle, and then rapidly design, test, and manufacture safe and efficacious vaccines. He believes that AdCEVTM/Egg technology can be used for rapid vaccine production at any scale. To him, that seems to be the best solution because it is fast, can be scaled linearly at any level of production from a single dose to hundreds of millions of doses, is a cost-effective manufacturing technique that answers the pressing needs of pandemic preparedness particularly in low- and middle-income countries, and is safe.
The best strategy to trigger a protective immune response against SARS-CoV 2 is to target a protein called a spike protein that surrounds the virus. However, just injecting coronavirus spike proteins is not the greatest technique to vaccinate people since spike proteins can be unstable and take on the wrong structure, causing the immune system to produce the incorrect antibodies. Dr. Blyden asserts that the AdCEVTM/Egg technology protein can be modified to make it more stable and preserve its shape, allowing it to withstand a COVID-19 infection and produce the necessary antibodies to fight it.
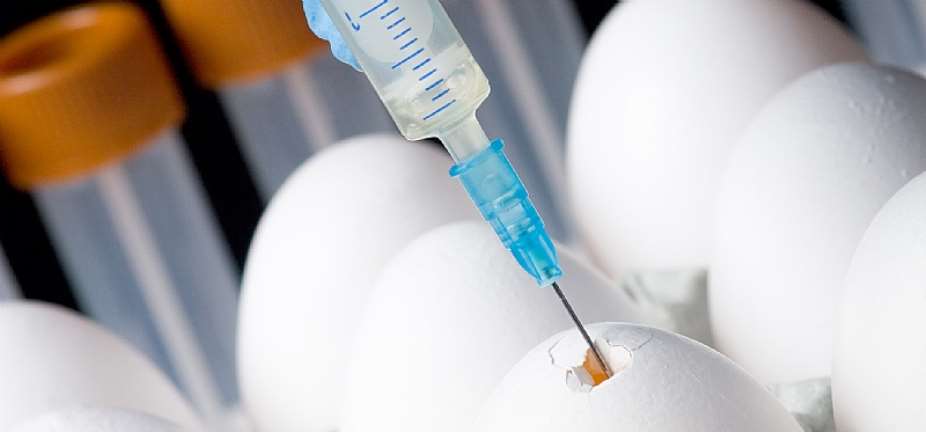
Every year, more than a billion doses of safe and effective egg-based flu vaccines are produced around the world, a sufficient capacity to switch to COVID-19 vaccine production. This method is reported to be safe for children, pregnant women, and the elderly. According to studies, the usual methods of egg-based vaccine production are pre-incubation of the eggs, inoculation with a live virus, incubation, harvesting of allantoic fluids, downstream processing, and filling and finishing. It can adapt to mutating viruses, has a track record of efficacy for critical infections, and can be manufactured in Africa. When compared to mRNA vaccines, which require super cold chains, temperature stability is an advantage that allows for administration in low-resource environments. Importantly, several vaccine manufacturers in Africa have been making egg-based vaccines and according to Dr. Blyden, African vaccine manufacturers already have the equipment and ability to develop, test, and deliver COVID-19 vaccines in massive amounts within a year of operationalization.
COVID-19 has highlighted Africa's lack of local manufacturing capacity, prompting African leaders to speed up local manufacturing expansion and encouraging some local manufacturers to commit to new vaccine projects. If the AdCEVTM/Egg technology for COVID-19 vaccines succeeds as expected, many countries will be able to vaccinate more individuals, bolstering local immunization and health systems in Africa and improving COVID-19 control and management of other infectious diseases.
Given the scale of COVID-19 vaccination take-up, the disease is unlikely to go away anytime soon, and many countries will need a continuous supply of vaccines to ensure individual protection and eventual herd immunity. African countries must come together, find investment opportunities, support investments into local vaccine development, and also invest in the development of the continent’s capacity to create the necessary infrastructural and human backbone to promote a healthy population at all times.
Author: Isabella Tetteh Ahinakwa (Host, The COVID-19 Diary)
REFERENCES
The World Health Organization (2022). The different types of COVID-19 vaccines (online). Available from: https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained [accessed 18th March 2022].
Vaccine factbook (2012). Basic Concept of Vaccination (online). Available from: http://www.phrma-jp.org/wordpress/wp-content/uploads/old/library/vaccine-factbook_e/1_Basic_Concept_of_Vaccination.pdf [accessed 18th March 2022].
BioPharm International (2010). New Approaches to Improved Vaccine Manufacturing in Embryonated Eggs (online). Available from: https://www.biopharminternational.com/view/new-approaches-improved-vaccine-manufacturing-embryonated-eggs [accessed 19th March 2022]
National Center for Biotechnology Information (2017). Vaccine Manufacturing (online). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7152262/ [accessed 20th March 2022]
The World Health Organization (2022). Eighth Meeting of the Multilateral Leaders Task Force on COVID-19, 1 March 2022: "Third Consultation with the CEOs of leading vaccine manufacturers" (online). Available from: https://www.who.int/news/item/07-03-2022-eighth-meeting-of-the-multilateral-leaders-task-force-on-covid-19-1-march-2022-third-consultation-with-the-ceos-of-leading-vaccine-manufacturers [accessed 18th March 2022]
Research Gate (2011). Poultry Pharming: Next-Generation Technologies for Egg-Based Biopharmaceutical Manufacturing (online). Available from: https://www.researchgate.net/profile/Eluemuno-Blyden/publication/221567913_Poultry_Pharming_Next-Generation_Technologies_for_Egg-Based_Biopharmaceutical_Manufacturing/links/60cae6e592851ca3aca747a1/Poultry-Pharming-Next-Generation-Technologies-for-Egg-Based-Biopharmaceutical-Manufacturing.pdf?origin=publication_detail [accessed 20th March 2022]
DCVMN member briefing – Presentation document (2021). Vaccine Manufacturing in Africa (online). Available from: https://www.dcvmn.org/IMG/pdf/20210316_vx_mf_africa_dcvmn_briefing_vpre-read.pdf [accessed 20th March 2022]




 We’ll protect state wealth from opaque deals – Prof Jane Naana
We’ll protect state wealth from opaque deals – Prof Jane Naana
 Mauritania president says running for second term in June polls
Mauritania president says running for second term in June polls
 I won't ever say I was a mere driver’s mate' — Prof. Opoku-Agyemang
I won't ever say I was a mere driver’s mate' — Prof. Opoku-Agyemang
 2024 polls: 'EC struggling to defend credibility'— Prof. Opoku-Agyemang
2024 polls: 'EC struggling to defend credibility'— Prof. Opoku-Agyemang
 Akufo-Addo gov't's 'greed, unbridled arrogance, unrestrained impunity, sheer dis...
Akufo-Addo gov't's 'greed, unbridled arrogance, unrestrained impunity, sheer dis...
 Election 2024: Ghana needs an urgent reset, a leadership that is inspiring – Ma...
Election 2024: Ghana needs an urgent reset, a leadership that is inspiring – Ma...
 Partner NDC to rollout a future of limitless prospects – Prof Jane Naana Opoku-A...
Partner NDC to rollout a future of limitless prospects – Prof Jane Naana Opoku-A...
 NPP will remain in gov’t till Jesus comes — Diana Asamoah
NPP will remain in gov’t till Jesus comes — Diana Asamoah
 Sunyani Technical University demands apology from former SRC president over sex-...
Sunyani Technical University demands apology from former SRC president over sex-...
 'Dumsor' was resolved by Mahama but ‘incompetent' Akufo-Addo has destroyed the g...
'Dumsor' was resolved by Mahama but ‘incompetent' Akufo-Addo has destroyed the g...
