Researchers at Janssen Pharmaceutical Companies are developing a Covid-19 vaccine candidate whose first human trials are expected to start in July. The team is eyeing early 2021 as a possible timeframe for release.
Roland Zahn, senior scientific director at Janssen, a subsidiary of Johnson & Johnson, is hopeful that despite the multiple challenges, his team will be able to develop the coronavirus vaccine in the first half of 2021.
“We are currently testing the vaccine in non-clinical animal studies to show the immunogenicity and efficacy of the vaccine candidate we have selected,” says Zahn.
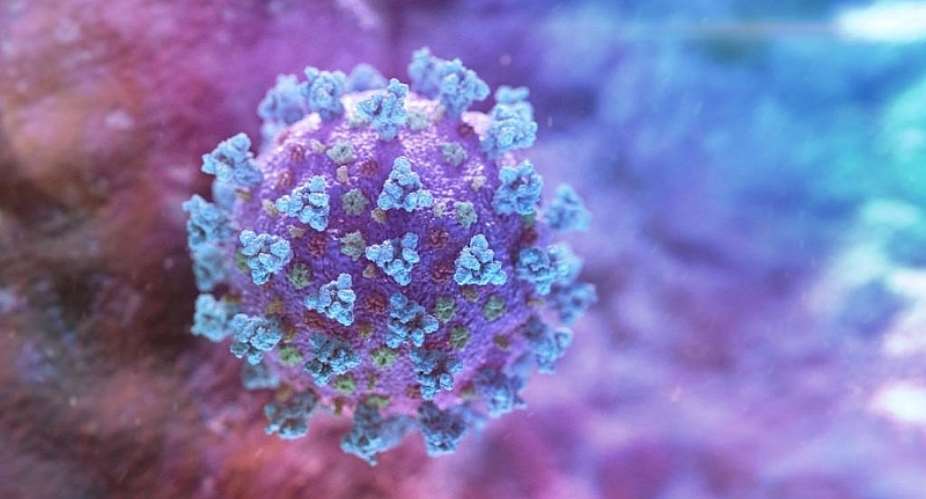
Their candidate vaccine uses an adenovirus, which normally causes a common cold, as a vector. To stop it replicating, the virus is made inactive by removing certain genes. In place, a piece of DNA that codes for the coronavirus spike protein is inserted. Once injected, these viral antigens are expressed in human cells that trigger the body's immune response.
The pharmaceutical giant aims to start clinical trials by July.
During phase 1, the safety of the vaccine candidate will be tested extensively. In this phase the company will also look at the immune response generated by the vaccine candidate.
As per the standard vaccine development procedure, Phase 2b/Phase 3 will be for testing its efficacy in the human population.
“Normally, vaccine development takes quite some time when we start first human trials. However, because of the Covid-19 pandemic, we could have an emergency use application where even if everything is not shown that normally has to be shown during a vaccine development, it is still possible to provide a vaccine.
“Of course, we still have to demonstrate the efficacy and the safety of the vaccine but certain aspects like consistency of manufacturing may not be required at this moment which is a must for a normal, licensed vaccine. In the case of the Covid-19, those studies can be done in parallel at a later stage,” Zahn said.
Moving fast
Developing a vaccine in around a year's time after an outbreak is unheard of. The challenges mount if a disease, like Covid-19, is new and therefore unknown.
“There is some precedence from the SARS and MERS outbreak that happened 16 years ago and is still ongoing in local places. However, those numbers are very low. Covid-19 is a different virus which has a higher mortality rate and its own particular disease symptoms.
“If the disease is unknown it makes it harder to understand what the antigen is and what part of the virus needs to be shown to the immune system so as to prevent the disease,” Zahn said.
He added that the other challenge is the speed at which things have to be moved for a Covid-19 vaccine.
“As we want to provide a vaccine as fast as possible, we have to do a lot of things in parallel. We have to produce a vaccine, test it in animals and scale up manufacturing, which is one of the most important steps. On a global scale, billions of doses are required,” he said.
- Indian firm to produce Covid-19 vaccine under clinical trial in Britain
- UK, Australia pour funds into coronavirus vaccine tests on humans
Promising technology
Zahn's optimism about the early 2021 timeframe stems from the fact that the vaccine is being developed with the same adenovirus vector technology used in the company's Ebola vaccine, as well as vaccine candidates for the Zika virus and HIV.
“Moreover, the antigen we are using has worked well so far in the human trials conducted by other vaccine developers like Moderna and the Oxford Group. This gives us more confidence that the antigen is also quite safe,” he said.
Janssen's vaccine candidate is one of the more than 100 Covid-19 vaccine candidates that are being developed the world over.




 We’ll no longer tolerate your empty, unwarranted attacks – TUC blasts Prof Adei
We’ll no longer tolerate your empty, unwarranted attacks – TUC blasts Prof Adei
 Bawumia donates GHc200,000 to support Madina fire victims
Bawumia donates GHc200,000 to support Madina fire victims
 IMF to disburse US$360million third tranche to Ghana without creditors MoU
IMF to disburse US$360million third tranche to Ghana without creditors MoU
 Truck owner share insights into train collision incident
Truck owner share insights into train collision incident
 Paramount chief of Bassare Traditional Area passes on
Paramount chief of Bassare Traditional Area passes on
 Two teachers in court over alleged illegal possession of BECE papers
Two teachers in court over alleged illegal possession of BECE papers
 Sunyani: Victim allegedly shot by traditional warriors appeals for justice
Sunyani: Victim allegedly shot by traditional warriors appeals for justice
 Mahama vows to scrap teacher licensure exams, review Free SHS policy
Mahama vows to scrap teacher licensure exams, review Free SHS policy
 Government will replace burnt Madina shops with a new three-story, 120-store fac...
Government will replace burnt Madina shops with a new three-story, 120-store fac...
