Bill Gates
The Bill & Melinda Gates Foundation on Monday announced that it will be supporting efforts in Guinea and other Ebola-affected countries to scale up the production and evaluation of convalescent plasma and other convalescent blood products as potential therapies for people infected with the Ebola virus.
Various drug candidates would also be evaluated, including the experimental antiviral drug brincidofovir.
In a press statement, the foundation said it has committed $5.7 million to launch the effort, and specific trial designs and locations would be confirmed in coordination with national health authorities and the World Health Organisation.
'We are committed to working with Ebola-affected countries to rapidly identify and scale up potential lifesaving treatments for Ebola,' Dr Papa Salif Sow, a senior programme officer and infectious diseases expert with the foundation's Global Health Programme, said.
'The Gates Foundation is focusing its R&D investments on treatments, diagnostics, and vaccines that we believe could be quickly produced and delivered to those who need them if they demonstrate efficacy in stopping the disease.'
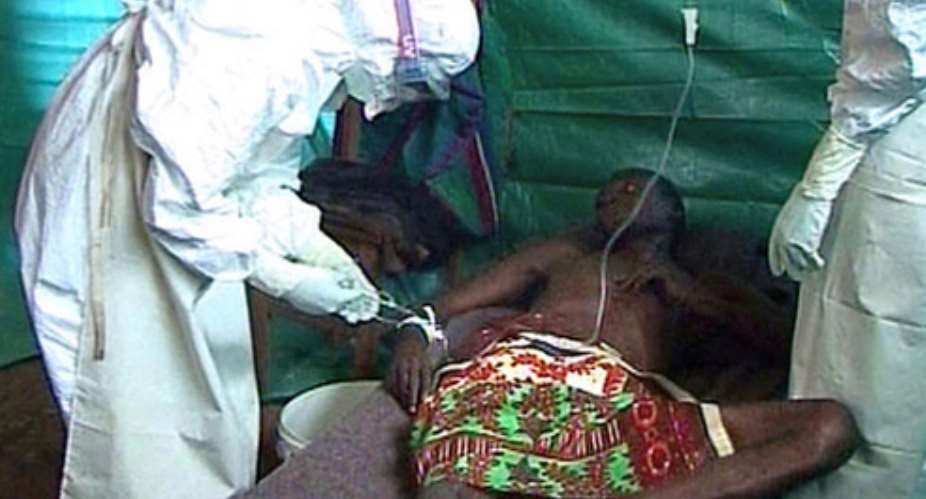
Convalescent plasma combined with pathogen inactivation technology represents a potential pathway to the development of an effective treatment for people infected with Ebola. The Gates Foundation is providing funding to Clinical Research Management, Inc. (ClinicalRM) and a broad array of private sector partners to study Ebola convalescent plasma that would be donated by Ebola survivors, in accordance with recent WHO guidance.
The convalescent plasma would be collected through mobile donation units fully equipped with Haemonetics PCS2â„¢ plasma collection systems and the Cerus INTERCEPTâ„¢ pathogen inactivation blood system.
Ebola survivors who are potential donors would be tested to ensure that they are cured of Ebola and are not infected with other blood-borne diseases. For added safety, the trial's use of pathogen inactivation technology would substantially reduce the risk of any transfusion-transmitted infection from blood components.
Until recently, convalescent plasma therapy was limited to transfusing whole blood collected from the survivor directly into the patient. Now, the best way to collect these antibodies is through plasmapheresis. This process uses a device to remove some blood from the donor and save only the liquid part, or plasma, containing antibodies.
The rest of the blood is returned to donors via infusion, which allows them to donate significantly more anti-Ebola antibodies than could be obtained via a whole blood donation. Use of plasmapheresis also allows survivors to donate every two weeks. By contrast, survivors who donate whole blood cannot volunteer for plasmapheresis for at least another three months, according to WHO guidance.




 SSNIT must be managed without gov’t interference – Austin Gamey
SSNIT must be managed without gov’t interference – Austin Gamey
 Ejisu by-election could go either way between NPP and independent candidate — Gl...
Ejisu by-election could go either way between NPP and independent candidate — Gl...
 We never asked ministers, DCEs to bring NPP apparatchiks for returning officer r...
We never asked ministers, DCEs to bring NPP apparatchiks for returning officer r...
 No one denigrated the commission when you appointed NDC sympathizers during your...
No one denigrated the commission when you appointed NDC sympathizers during your...
 Used cloth dealers protests over delayed Kumasi Central Market project
Used cloth dealers protests over delayed Kumasi Central Market project
 A/R: Kwadaso onion market traders refuse to relocate to new site
A/R: Kwadaso onion market traders refuse to relocate to new site
 Dumsor: Corn mill operators at Kaneshie market face financial crisis
Dumsor: Corn mill operators at Kaneshie market face financial crisis
 Jamestown fishermen seek support over destruction of canoes by Tuesday's heavy d...
Jamestown fishermen seek support over destruction of canoes by Tuesday's heavy d...
 Election 2024: EC to commence voter registration exercise on May 7
Election 2024: EC to commence voter registration exercise on May 7
 Public schools rebranding: We’re switching to blue and white, we’re painting all...
Public schools rebranding: We’re switching to blue and white, we’re painting all...
