As the world scrambles to find a vaccine to fight Covid-19, which has killed at least 170,000 people, Germany and the UK are set to begin human testing.
Although at least 120 projects around the world are working towards a vaccine, just 5 clinical trials on humans have so far been approved.
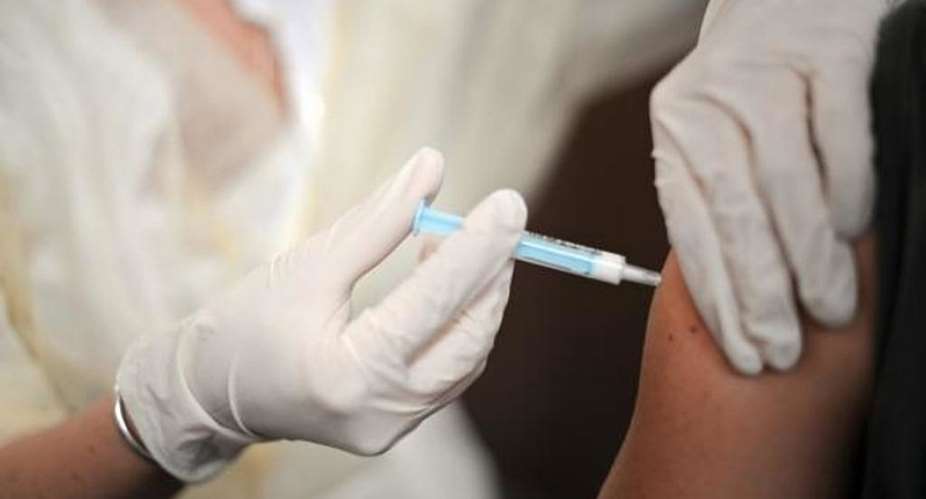
Researchers from the University of Oxford on Thursday began trialling the first dose of a potential vaccine based on a virus found in chimpanzees. It will involve 510 healthy volunteers aged between 18 and 55 and has been given an 80 percent chance of success.
Meanwhile the German biotech company Biontech will become the first European business to proceed with clinical trials of a Covid-19 vaccine, which it has been working on a vaccine since January.
Four variants of a prototype developed with US pharma giant Pfizer will be tested on 200 volunteers also aged between 18 and 55. German regulatory body PEI, which approved the human trials, which will begin at the end of April, said they marked a “significant step” in making a vaccine available as soon as possible.
Most trials still in early stages
The vast majority of the world's vaccine projects, including those developed in France by Sanofi and the Institute Pasteur, are in the so-called preclinical phase.
"It is during this stage that we develop a prototype according to the type of immune reaction that we want to induce in the body", former World Health Organisation assistant director Marie-Paule Kieny told France Info.
"Once the prototype is found, we test on small animals, often mice, and we produce a vaccine batch that meets all the standards in clinical use and passes all regulatory controls for human administation.”
This step takes at least two months, adds Kieny, a French virologist who sits on a committee advising the government on treatments and tests to combat the virus that causes Covid-19.
- Is it possible the BCG vaccine protects against the new coronavirus?
- World Earth Day stresses the link between health and environment
Meanwhile a study published by the Institut Pasteur suggests only 5.7 percent of the country's population will have been infected by the time lockdown measures are eased on 11 May. At least 60 percent is considered necessary to achieve herd immunity.
Experts estimate that it will take at least 12 to 18 months to develop a Covid-19 vaccine. So far there are no approved medication for the disease, which has infected more than two million people.




 Dumsor: Energy sector ‘shepherdless’ – Nana Amoasi VII
Dumsor: Energy sector ‘shepherdless’ – Nana Amoasi VII
 Train accident: Four more grabbed and remanded
Train accident: Four more grabbed and remanded
 Gov't to consolidate cash waterfall revenue collection accounts
Gov't to consolidate cash waterfall revenue collection accounts
 Gov't to settle lump sum for retired teachers by April 27
Gov't to settle lump sum for retired teachers by April 27
 Former PPA CEO granted GH₵4million bail
Former PPA CEO granted GH₵4million bail
 Dumsor: The darkness has exposed you; you’ll go down as the worst in Ghana’s his...
Dumsor: The darkness has exposed you; you’ll go down as the worst in Ghana’s his...
 Dumsor: The ‘incompetent’ person provided a timetable whiles those who came to s...
Dumsor: The ‘incompetent’ person provided a timetable whiles those who came to s...
 Defend, ensure NPP’s good works are ‘sold’ and highlight the ‘bad’ state of the ...
Defend, ensure NPP’s good works are ‘sold’ and highlight the ‘bad’ state of the ...
 Bawumia will rank high ahead of Mahama in any anti-corruption test — Salam Musta...
Bawumia will rank high ahead of Mahama in any anti-corruption test — Salam Musta...
 NPP trying to bribe us but we‘ll not trade our integrity on the altar of corrupt...
NPP trying to bribe us but we‘ll not trade our integrity on the altar of corrupt...
