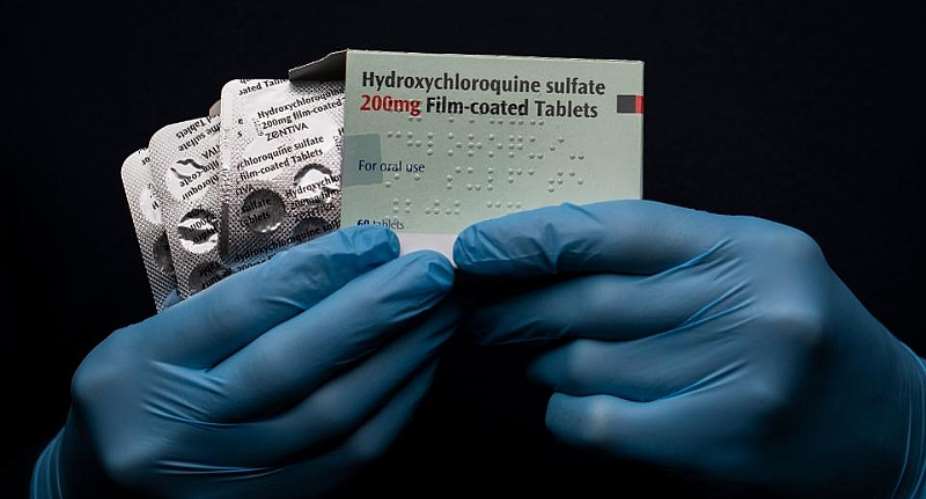
Importers are being allowed to bring in more stocks of hydroxychloroquine to help treat coronavirus cases in Ghana.
At a press conference on Wednesday morning, Minister of Health, Kwaku Agyemang Manu, said he had given authorisation to the Food and Drugs Authority (FDA) to allow its use despite it not being on Ghana’s drugs list.
“On mentioning hydroxychloroquine, I just want to inform the nation that the laws of our country allow the Health Minister to issue what we call emergency use authorisation to FDA to allow or permit manufactures and importers to bring in drugs that are not on our drug list,” Mr Agyemang Manu explained.
“We issued the authorisation and the FDA has on that note, permitted some manufacturers in the country to bring in the active pharmaceutical ingredients or even import if they can source from outside to augment the stocks we have in the country.”
At the moment, he said the country had enough hydroxychloroquine stocks “to take care of all the people we have on admission.”
About Hydroxychloroquine use
Following its discovery in 1934, Chloroquine was believed to be too toxic for human use.
But it was introduced into clinical practice in 1947 for the preventive treatment of malaria.
The use of chloroquine for treating malaria has reduced over the years because of its side effects and increased resistance of the malaria parasite.
Hydroxychloroquine, which is a less toxic derivative of chloroquine, has in recent times become more preferred because of its better safety record and cheaper cost.
In the wake of the coronavirus pandemic, a new study whose results were published in the International Journal of Antimicrobial Agents has found some evidence that the combination of hydroxychloroquine and an antibiotic called azithromycin could be especially effective in treating the novel coronavirus.
America’s Food and Drug Administration recently issued emergency authorisation for experimental coronavirus treatments using chloroquine and hydroxychloroquine.
But some other health experts provided mixed assessments warning of risks such as vision problems or cardiac arrest.
---citinewsroom




 This IMANI job no dey pap; the people you are fighting for are always fighting y...
This IMANI job no dey pap; the people you are fighting for are always fighting y...
 Prof. Naana Opoku-Agyemang has changed; you can see a certain sense of urgency –...
Prof. Naana Opoku-Agyemang has changed; you can see a certain sense of urgency –...
 MFWA Executive Director slams Akoma FM for engaging in ‘irresponsible’ media pra...
MFWA Executive Director slams Akoma FM for engaging in ‘irresponsible’ media pra...
 ‘Women must become millionaires too’ — Prof Jane Naana on establishment of Women...
‘Women must become millionaires too’ — Prof Jane Naana on establishment of Women...
 Some believe only in Ghanaian votes, not Ghana — Kofi Asare jabs politicians
Some believe only in Ghanaian votes, not Ghana — Kofi Asare jabs politicians
 Plan to make BEST sole aggregator of Sentuo Oil Refinery will create market chal...
Plan to make BEST sole aggregator of Sentuo Oil Refinery will create market chal...
 2024 elections: I can't have the man I removed from office as my successor — Aku...
2024 elections: I can't have the man I removed from office as my successor — Aku...
 2024 Elections: Immediate-past NPP Germany Branch Chairman garners massive votes...
2024 Elections: Immediate-past NPP Germany Branch Chairman garners massive votes...
 Gov’t focused on making Ghana energy self-sufficient, eco-friendly – Akufo-Addo
Gov’t focused on making Ghana energy self-sufficient, eco-friendly – Akufo-Addo
 April 25: Cedi sells at GHS13.74 to $1, GHS13.14 on BoG interbank
April 25: Cedi sells at GHS13.74 to $1, GHS13.14 on BoG interbank
