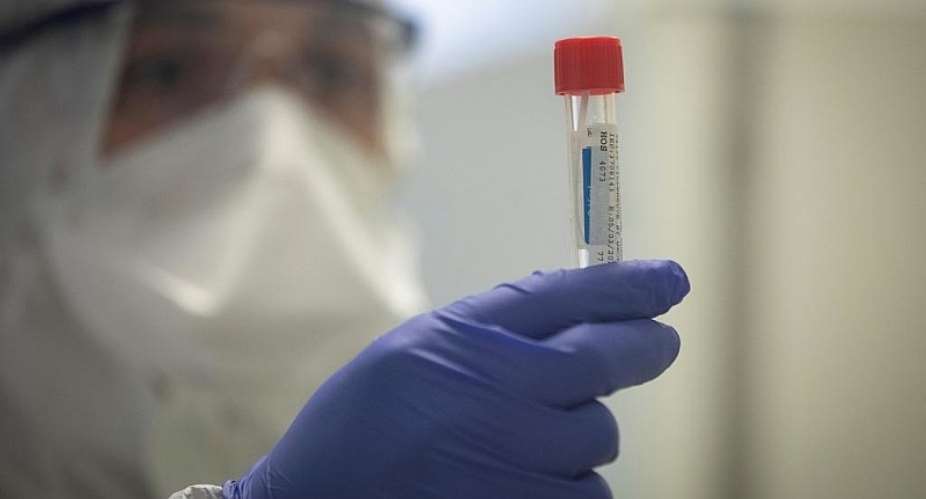
The novel coronavirus continues its expansion across the world with more than 255,000 cases and 10,000 deaths, as of mid-Friday. The figures rise every hour. Racing against that clock, some 20 biotechnology companies and organisations, mostly in the US, Europe and China, are working to find a vaccine and treatment for Covid-19.
Massachussets-based company Moderna is testing its first vaccines against Covid-19 in the US and has taken the lead - for now...
While Chinese scientists are researching multiple types of vaccines simultaneously in hospitals and universities across the country.
• United States
Moderna
Vaccine: "mRNA-1273"
1st clinical test in Seattle on March 16th on 45 healthy patients
Forecast: Available in 12-18 months if efficient
(Source: Food and Drug administration/FDA)
Gilead Sciences
"Remdesivir" could be the first treatment on the market
Used to treat Ebola but not efficient
Forecast: Available in the coming months
(Source: Bruce Aylward, World Health Organization/WHO)
Inovio Pharmaceuticals
DNA vaccine
Fist clinical tests in April in the US, then in China and South Korea
Forecast: delivering 1 million doses by the end of the year
(Source: J. Joseph Kimn, CEO Inovio)
• Germany
CureVac
DNA vaccine
The European Commission offered 80 million euros to CureVac on 17 March to develop the vaccine
A candidate vaccine could be provided within a few months
(Source: CEO, CureVac)
• France
Pasteur Institute
Use of measles vaccine
Forecast: first vaccine by fall 2020
Pasteur institute to be given 5 million euros to develop vaccine
(Source: Pasteur Institute)
Sanofi
In cooperation with US Health Ministry
DNA vaccine
Possible clinical test within 12-18 months
(Source: David Loew, Vice-president Sanofi Pasteur)
IHU – Institut hospitalo-universitaire
Treatment with chloroquine (anti-malaria drug)
Has been testing since 9 March on 24 patients; after 6 days, only 25% of patients are still infected (Source: Pr. Didier Raoult, IHU Director)
French Health ministry expresses methodological concerns but has given green light to larger clinical tests
Sanofi has offered 300,000 anti-malaria doses if proven efficient
• China
Clover Biopharmaceuticals
Chengdu-based
Partnership with GSK (Bratford, UK)
GSK will provide Clover with its proprietary adjuvants
Chinese scientists researching multiple types of vaccines simultaneously
Some have entered animal trials (mice)
Forecast: first clinical trials in late April 2020
(Source: Chinese National Health Commission)
• Japan
Fujifilm Biotechnologies
Flu shot treatment Avigan
Tested on board of the Diamond Princess ship cruise when in quarantine
Clinical tests on 200 patients in Wuhan and Shenzen, China
(Source: Chinese National Health Commission)




 Whoever participated in the plunder of the state must be held accountable – Jane...
Whoever participated in the plunder of the state must be held accountable – Jane...
 A vote for John and Jane is a vote to pull Ghana from the precipice of destructi...
A vote for John and Jane is a vote to pull Ghana from the precipice of destructi...
 I’ll repay your abiding confidence with loyalty, understanding and a devotion to...
I’ll repay your abiding confidence with loyalty, understanding and a devotion to...
 ‘I’ve learnt deeply useful lessons for the future' — Serwaa Amihere breaks silen...
‘I’ve learnt deeply useful lessons for the future' — Serwaa Amihere breaks silen...
 I’m sorry for the embarrassment – Serwaa Amihere apologises for leaked sex video
I’m sorry for the embarrassment – Serwaa Amihere apologises for leaked sex video
 Dumsor: Matthew Opoku Prempeh not in charge of Energy sector – Minority
Dumsor: Matthew Opoku Prempeh not in charge of Energy sector – Minority
 Adu Boahen’s murder: Police arrest house help who was in possession of deceased’...
Adu Boahen’s murder: Police arrest house help who was in possession of deceased’...
 Akufo-Addo nominates Felicia Attipoe as Tema West MCE
Akufo-Addo nominates Felicia Attipoe as Tema West MCE
 Election 2024: I can't have someone I defeated twice as my successor – Akufo-Add...
Election 2024: I can't have someone I defeated twice as my successor – Akufo-Add...
