Uganda has fast-tracked testing of Ebola vaccines, as the WHO declared the outbreak in neighboring Democratic Republic of Congo a "public health emergency of international concern", a rare designation only used for the gravest epidemics.
Uganda lies just across the border with the DRC where more than 1,600 people have died from the outbreak since August 2018. More than 2,500 cases have been confirmed.
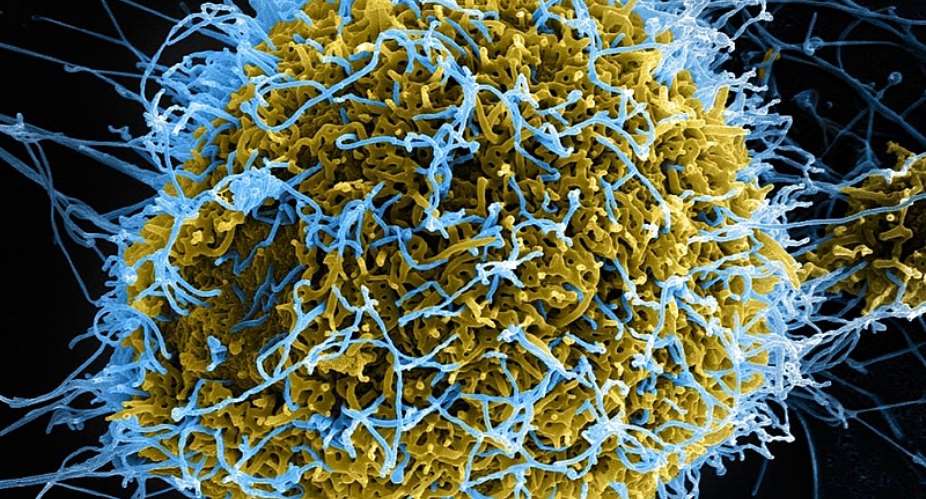
The strain of the Ebola virus targeted by the vaccine is codenamed "Sudan". It's one of five known strains of the virus that struck Uganda in 2012 killing 17 out of 24 cases confirmed during the outbreak.
The Kampala-based Independent newspaper reports that the government is looking to shield thousands of health workers putting their lives at risk every day along its border with the DRC.
Uganda has so far vaccinated over 6,000 health and frontline workers with the only known experimental Ebola-rV5V vaccine that was produced by Merck Pharmaceuticals.
Clinical trials
The Independent says the vaccine developed by the American Vaccine Research Center and the National Institute of Health in Maryland, will be tested on some 40 health workers over a period of 48 weeks.
The trials come just days after the confirmation of the first Ebola case in Goma, a city of about one million people located on the northern shore of Lake Kivu, bordering Rwanda.
While more than 160,000 people in the affected provinces of North Kivu and Ituri have been vaccinated, containment efforts have been hampered by chronic unrest in the region and a lack of trust in communities for health workers.
Goma case 'game-changer'
WHO chief Tedros Adhanom Ghebreyesus, who held off declaring the outbreak in the North Kivu an emergency on three previous occasions, described the outbreak as a “potential game-changer”.
War-ravaged Goma is the strategic gateway to Africa's Great Lakes region and the wider world.
Ghebreyesus is reported to have accepted the advice of his advisory board to invoke the “emergency” provision due to the high risks of further outbreaks in DRC cities, with high population density and mobility.
That, according to the WHO, will make it far harder to isolate Ebola patients and trace their contacts.
“These are desperate times because the government has been struggling without hope," regrets Joseph Were, editor of the Independent.
Were told RFI that Uganda's main concern is not for the safety of health workers on the frontline who are vaccinated, but the wider population that remains at great risk, especially at big border markets frequently visited by up to 10,000 people crossing into the country from the DRC.
Compassionate arrangements
The new Ebola Sudan vaccine, developed and sponsored by several American agencies including the Maryland-based National Institute of Health, has not been approved for licensing by the WHO.
But according to Dr Bernard Opar, Principal Medical Officer at Uganda's Health Ministry in Kampala, the UN agency's Strategic Advisory Group of Experts (SAGE), had granted Uganda the permission to test it, “under a compassionate arrangement”.
“SAGE recommends that the vaccine be administered under experimental conditions since Ebola has no cure”, on the basis that “it can provide help in the absence of none”.
Dr Opar however remains hopeful about Uganda's efforts to contain the 'Zaire' strain of the virus. This is while researchers at Mbarara University of Science and Technology get ready to experiment another vaccine developed by Janssen Pharmaceuticals.
Funding delays
With stakeholders locked in a race against time to stop the spread of Ebola, the panel of top WHO officials which declared the outbreak in the DRC an emergency also expressed "disappointment at delays in funding, which members claimed had “constrained the response".
The Geneva-based organisation warned that a fresh UN funding appeal for 700 million dollars expected in coming days was only sufficient to cover the ensuing six months.




 Meta releases new version of conversational AI across its platforms
Meta releases new version of conversational AI across its platforms
 Cape Town named Africa’s Best Airport 2024 by Skytrax
Cape Town named Africa’s Best Airport 2024 by Skytrax
 Bono East: Four injured after hearse transporting corpse crashes into a truck
Bono East: Four injured after hearse transporting corpse crashes into a truck
 ‘Be courageous, find your voice to defend our democracy’ — Sam Jonah urges journ...
‘Be courageous, find your voice to defend our democracy’ — Sam Jonah urges journ...
 Exodus of doctors, nurses and teachers have worsened because of unserious Akufo-...
Exodus of doctors, nurses and teachers have worsened because of unserious Akufo-...
 2024 election: Avoid insults, cutting down people in search of power – National ...
2024 election: Avoid insults, cutting down people in search of power – National ...
 ‘You passed through the back door but congratulations’ — Atubiga on Prof Jane Na...
‘You passed through the back door but congratulations’ — Atubiga on Prof Jane Na...
 Government’s $21.1 billion added to the stock of public debt has been spent judi...
Government’s $21.1 billion added to the stock of public debt has been spent judi...
 Akufo-Addo will soon relocate Mahama’s Ridge Hospital to Kumasi for recommission...
Akufo-Addo will soon relocate Mahama’s Ridge Hospital to Kumasi for recommission...
 We must not compromise on our defence of national interest; this is the time to ...
We must not compromise on our defence of national interest; this is the time to ...
