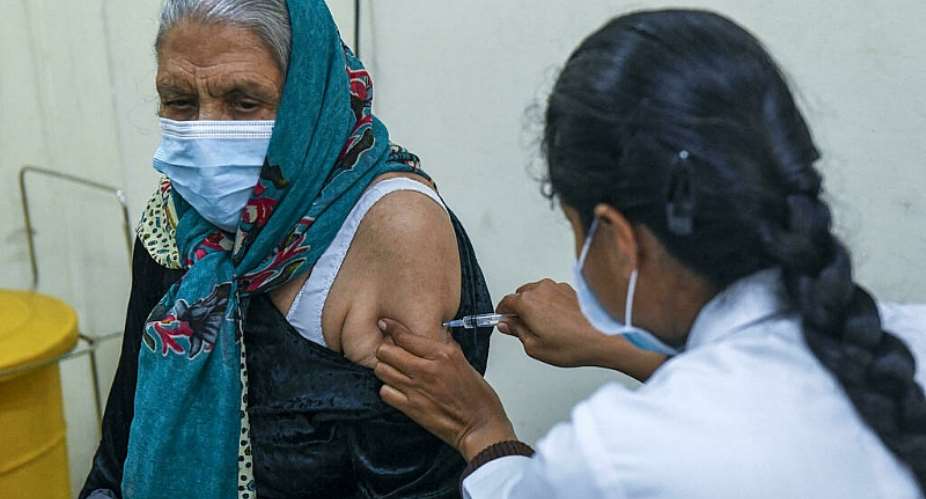
An Indian drug firm has promised an Omicron-specific vaccine that it says is “safe, tolerable and immunogenic”. The move comes as the new variant spreads across the country, where millions of people have yet to get their Covid shots.
India reported 317,532 new infections on Thursday, taking the number of cases so far to 38 million, with 487,000 deaths.
Privately-run Gennova Biopharmaceuticals said it has sent the basic data of its Omicron vaccine to the Central Drugs Standard Control Organisation, a federal regulator.
“The Omicron-specific variant of vaccine is under development and will be ready for human clinical trials, subject to regulatory approvals," media quoted a company spokesperson as saying.
Late stage trials of the Omicron vaccine could begin in February after an official clearance expected in the next few days.
Safety assurances
Gennova, based in Maharashtra state, asserted the federal regulator in an initial study certified the two-dose messenger or mRNA vaccine “safe, tolerable and immunogenic.”
“It is a thing of pride that Omicron was declared on 25 November in the world and within one and a half months we are getting this vaccine,” said Avinash Bhondwe, former chief of the Maharashtra chapter of Indian Medical Association.
Bhondwe told television the under-trial vaccine “was giving more than 80 percent of protection” against Omicron.
The firm has started producing the vaccine and would roll out “sufficient quantities” after the government's green flag, others added.
If approved, it would be India's first mRNA vaccine.
V.K. Paul, the government's top coronavirus adviser, said the Gennova product could prove useful beyond Covid.
“The development of this vaccine is a significant achievement for the country,” Paul added.
“The Omicron-specific vaccine under development is also exciting while the quick-switch platform would come into play as and when mutant strains emerge,” the government advisor was quoted as saying.
Pfizer has said a re-crafted vaccine to tackle omicron was likely to be ready for launch in March and that the US-based firm had already begun the production.
Vaccine anniversary
The development came as India marked the first anniversary of its health drive by fully inoculating 70 percent of its 1.3 billion people with Oxford-AstraZeneca shots and a local vaccine.
India has given 1.6 billion doses but a population the size of South Africa's is yet to receive the first shot. The government told courts vaccination was not mandatory even though infections spiked six-fold in the past week.
India however logged only 9,287 Omicron infections by Thursday, giving rise to speculation the real count would be vastly higher as people diagnosed positive at home were not reporting back.
“Home testing kits are selling like hot cakes as prescriptions are not needed for the product,” said Vinod, a drug store owner in a Delhi suburb who uses one name.
Chest doctor Sushmita Roychowdhury from the Fortis healthcare chain suggested a “temporary law” to compel people to wear face masks in public.
And only then “all this questions will go away because we don't know whether we are treating Delta or Omicron,” the Kolkata-based doctor told a public discussion.
Hemant Thacker from a state Covid task force joined the call, seeking stiff action in public interest as new infections in India spiked by 1.63 million in six days to 20 January.
“You must ask for the heaviest (of) fines so that the country and the community can be saved,” the doctor said as four Supreme Court judges tested positive.
Several cities have ordered on-spot cash penalties for not masking up while policemen in some states depend on their night sticks to enforce public hygiene.




 This IMANI job no dey pap; the people you are fighting for are always fighting y...
This IMANI job no dey pap; the people you are fighting for are always fighting y...
 Prof. Naana Opoku-Agyemang has changed; you can see a certain sense of urgency –...
Prof. Naana Opoku-Agyemang has changed; you can see a certain sense of urgency –...
 MFWA Executive Director slams Akoma FM for engaging in ‘irresponsible’ media pra...
MFWA Executive Director slams Akoma FM for engaging in ‘irresponsible’ media pra...
 ‘Women must become millionaires too’ — Prof Jane Naana on establishment of Women...
‘Women must become millionaires too’ — Prof Jane Naana on establishment of Women...
 Some believe only in Ghanaian votes, not Ghana — Kofi Asare jabs politicians
Some believe only in Ghanaian votes, not Ghana — Kofi Asare jabs politicians
 Plan to make BEST sole aggregator of Sentuo Oil Refinery will create market chal...
Plan to make BEST sole aggregator of Sentuo Oil Refinery will create market chal...
 2024 elections: I can't have the man I removed from office as my successor — Aku...
2024 elections: I can't have the man I removed from office as my successor — Aku...
 2024 Elections: Immediate-past NPP Germany Branch Chairman garners massive votes...
2024 Elections: Immediate-past NPP Germany Branch Chairman garners massive votes...
 Gov’t focused on making Ghana energy self-sufficient, eco-friendly – Akufo-Addo
Gov’t focused on making Ghana energy self-sufficient, eco-friendly – Akufo-Addo
 April 25: Cedi sells at GHS13.74 to $1, GHS13.14 on BoG interbank
April 25: Cedi sells at GHS13.74 to $1, GHS13.14 on BoG interbank
