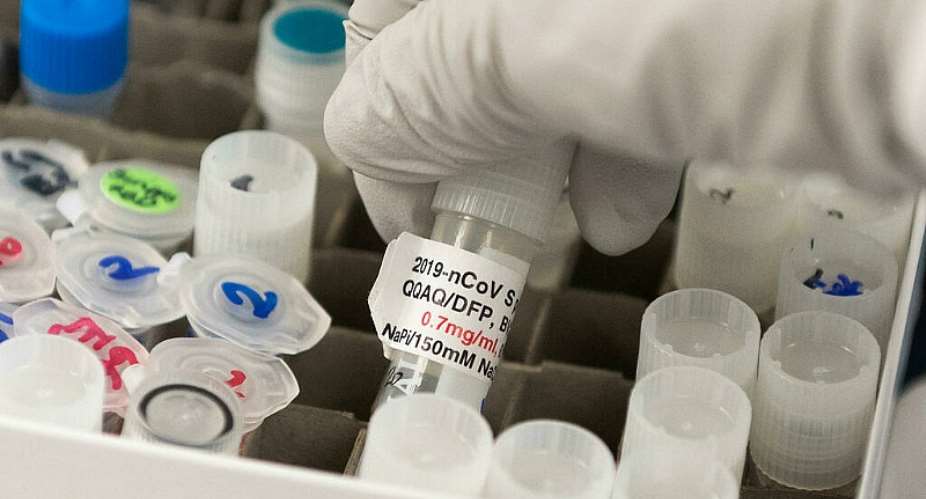
The European Commission has signed a preliminary deal to buy up to 200 million doses of a promising Covid-19 vaccine from US pharmaceutical firm Novavax. The agreement depends on the Novavax vaccine being approved by the EU's regulator, the European Medicines Agency.
Making the announcement, EU health commissioner Stella Kyriakides said: "Our new agreement with Novavax expands our vaccine portfolio to include one more protein-based vaccine, a platform showing promise in clinical trials."
If approved, the Novavax jab would slot in alongside the EU's Covid-19 vaccines already in use: these include a shot from BioNTech/Pfizer, which forms the backbone of the European vaccination drive; as well as those from AstraZeneca, Moderna and Johnson & Johnson.
The 27-nation European Union, after a faltering start early this year, has now fully vaccinated 50.7 percent of its population of 445 million, according to a French news agency tally of official national figures.
4.57 billion, 445 million people?
With the latest deal, the bloc has secured access to up to 4.57 billion doses of Covid vaccines from seven different suppliers.
BioNTech/Pfizer's vaccine is by far the biggest joint purchase by the EU, amounting to more than half that total, 2.4 billion doses.
The portfolio includes vaccines from three pharmaceutical companies that have still not received approval from the European Medicines Agency at this time: along with the Novavax product there are one each from CureVac and GSK-Sanofi.
Unlike the mRNA technology used by BioNTech/Pfizer, Moderna and Curevac, Novavax's two-jab vaccine relies on a traditional technique, using proteins to carry fragments of the coronavirus rendered harmless to produce an immune reaction.
This means it does not have to be stored in ultra-low temperatures, potentially giving it a logistical edge.
90 percent efficacy
Novavax, a company based in the US state of Maryland, says its vaccine has 90 percent efficacy against Covid-19, based on a North American study. US and EU regulators have not yet given their evaluation of the jab's efficacy.
The deal announced on Wednesday would secure the EU a firm purchase of 100 million doses of the Novavax vaccine, with an option for 100 million more, once EMA approval was given. Deliveries would stretch from this year into 2023.
The EU commission said that, under the contract, member states "will also be able to donate vaccines to lower and middle-income countries or to re-direct them to other European countries".




 This IMANI job no dey pap; the people you are fighting for are always fighting y...
This IMANI job no dey pap; the people you are fighting for are always fighting y...
 Prof. Naana Opoku-Agyemang has changed; you can see a certain sense of urgency –...
Prof. Naana Opoku-Agyemang has changed; you can see a certain sense of urgency –...
 MFWA Executive Director slams Akoma FM for engaging in ‘irresponsible’ media pra...
MFWA Executive Director slams Akoma FM for engaging in ‘irresponsible’ media pra...
 ‘Women must become millionaires too’ — Prof Jane Naana on establishment of Women...
‘Women must become millionaires too’ — Prof Jane Naana on establishment of Women...
 Some believe only in Ghanaian votes, not Ghana — Kofi Asare jabs politicians
Some believe only in Ghanaian votes, not Ghana — Kofi Asare jabs politicians
 Plan to make BEST sole aggregator of Sentuo Oil Refinery will create market chal...
Plan to make BEST sole aggregator of Sentuo Oil Refinery will create market chal...
 2024 elections: I can't have the man I removed from office as my successor — Aku...
2024 elections: I can't have the man I removed from office as my successor — Aku...
 2024 Elections: Immediate-past NPP Germany Branch Chairman garners massive votes...
2024 Elections: Immediate-past NPP Germany Branch Chairman garners massive votes...
 Gov’t focused on making Ghana energy self-sufficient, eco-friendly – Akufo-Addo
Gov’t focused on making Ghana energy self-sufficient, eco-friendly – Akufo-Addo
 April 25: Cedi sells at GHS13.74 to $1, GHS13.14 on BoG interbank
April 25: Cedi sells at GHS13.74 to $1, GHS13.14 on BoG interbank
