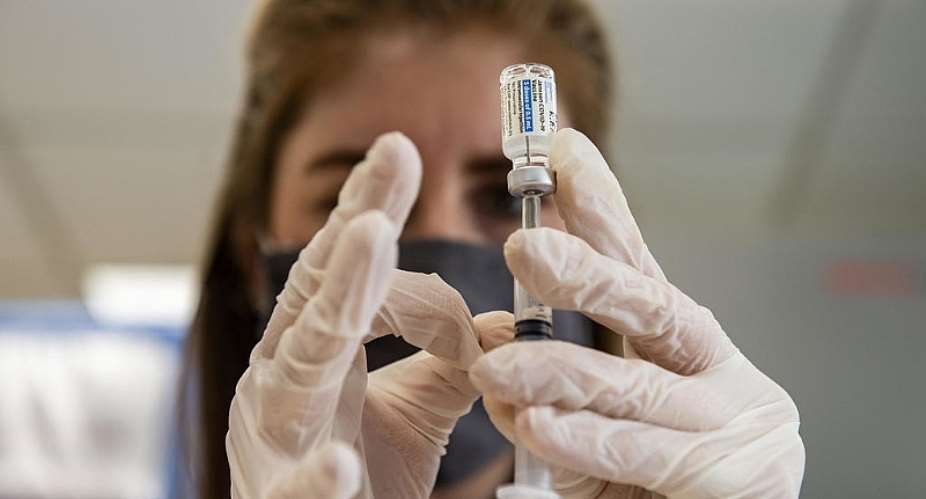
Denmark has said it will not use US drugmaker Johnson & Johnson's single-shot Covid-19 vaccine in its vaccination campaign, citing concerns over serious side effects involving blood clots.
"The Danish Health Authority has concluded that the benefits of using the Covid-19 vaccine from Johnson & Johnson do not outweigh the risk of causing the possible adverse effect...in those who receive the vaccine," the authority said in a statement.
"Therefore, the Danish Health Authority will continue the Danish mass vaccination programme against Covid-19 without the Covid-19 vaccine from Johnson & Johnson," it added.
The World Health Organization and European medicines watchdog have both authorised the J&J vaccine.
According to TV2 News, Health Minister Magnus Heunicke, informed Parliament on Monday that the vaccine would be taken out of the Danish inoculation programme based on recommendations by the local health authority.
'Waiting until Christmas'
The decision means that a last age group of Danes will likely have to wait until the autumn to be fully vaccinated when other authorised vaccines become available to them.
The Danish weekly CPHPost quoted Professor Jan Pravsgaard Christensen from the Department of Immunology and Microbiology at the University of Copenhagen as saying that if only Pfizer and Moderna (the only vaccines allowed by Danish authorities) are to be applied, the last group "will have to wait until Christmas".
As was the case with the AstraZeneca jab, the vaccine will be axed due to concerns relating to serious blood clots.
Denmark has not used the J&J vaccine yet, but it does have doses in storage. In total, Denmark was scheduled to receive 8.2 million doses, which are now "down the toilet", CPHPost wrote.
The vaccine may return to the vaccination programme at some point as part of a voluntary scheme, says the newspaper.
Last month, J&J confirmed that they had “reviewed the cases with the European health authorities, and we have chosen to postpone the rollout of the vaccine in Europe”, while the US Food and Drug Administration started new studies to assess whether it is still safe to use the vaccine.
France began administering the J&J vaccine on 24 April.




 Former Kotoko Player George Asare elected SRC President at PUG Law Faculty
Former Kotoko Player George Asare elected SRC President at PUG Law Faculty
 2024 elections: Consider ‘dumsor’ when casting your votes; NPP deserves less — P...
2024 elections: Consider ‘dumsor’ when casting your votes; NPP deserves less — P...
 You have no grounds to call Mahama incompetent; you’ve failed — Prof. Marfo blas...
You have no grounds to call Mahama incompetent; you’ve failed — Prof. Marfo blas...
 2024 elections: NPP creates better policies for people like us; we’ll vote for B...
2024 elections: NPP creates better policies for people like us; we’ll vote for B...
 Don’t exchange your life for wealth; a sparkle of fire can be your end — Gender ...
Don’t exchange your life for wealth; a sparkle of fire can be your end — Gender ...
 Ghana’s newly installed Poland train reportedly involved in accident while on a ...
Ghana’s newly installed Poland train reportedly involved in accident while on a ...
 Chieftaincy disputes: Government imposes 4pm to 7am curfew on Sampa township
Chieftaincy disputes: Government imposes 4pm to 7am curfew on Sampa township
 Franklin Cudjoe fumes at unaccountable wasteful executive living large at the ex...
Franklin Cudjoe fumes at unaccountable wasteful executive living large at the ex...
 I'll 'stoop too low' for votes; I'm never moved by your propaganda — Oquaye Jnr ...
I'll 'stoop too low' for votes; I'm never moved by your propaganda — Oquaye Jnr ...
 Kumasi Thermal Plant commissioning: I pray God opens the eyes of leaders who don...
Kumasi Thermal Plant commissioning: I pray God opens the eyes of leaders who don...
