India's first locally-developed Covid-19 vaccine posted 81 percent interim efficacy in late stage trials involving nearly 26,000 people, putting to rest doubts and debate over the shots and brightening prospects of its export to Brazil, and other countries.
Bharat Biotech, the manufacturer, hailed the verdict as "an important milestone in vaccine discovery, for science and our fight against coronavirus" and said the exercise involved the largest cluster of volunteers in a clinical trial in India.
The vaccine jointly developed by Biotech and state-run Indian Council of Medical Research was authorized for emergency use in January even while the trials were underway.
The report also drew praise from public health experts such as Randeep Guleria, who heads India's largest hospital in Delhi.
“The interim result as it is right now is very, very encouraging and I think it should put all sort of doubts at rest,” he said, adding that Covaxin must be “promoted” since India had an enormous task in hand.
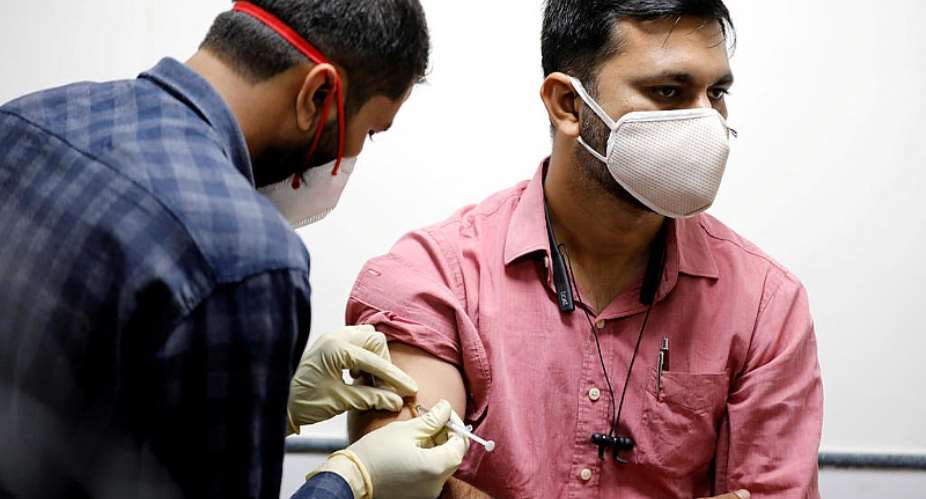
India in January rolled out its phased campaign to inoculate 1.35 billion citizens against coronavirus.
Shot in the arm
India plans to inoculate 300 million people by the end of July and analysts said the publication of Covaxin's efficacy report would be a 'shot in the arm' for the world's largest vaccination drive.
Prime Minister Narendra Modi took his first Covid shot on Monday and urged Indians to unite against the virus.
Cabinet ministers such as foreign minister Subrahmanyam Jaishankar also extended support to the local vaccine as many of them took their first Covaxin jabs.
Minimal resistance to vaccination
Dhruva Choudhry, a pulmonologist who leads a provincial Covid-19 vaccine task-force argued there was no reason to have any doubts over Covaxin.
“With the prime minister taking the vaccine, all the top luminaries in the government, in the medical establishment taking the Covaxin, that particular component needs to go away that this is not an effective vaccine,” he told state-run RSTV television.
Narendra Kumar Arora of a national advisory group on immunization argued only a handful of healthcare personnel had refused to accept the vaccine.
“During last six weeks, 15 million people have been immunized and this itself is a big scientific proof that the vaccine is safe,” Arora asserted.
A vaccine developed by British pharmaceutical firm AstraZeneca is the other virus shot currently available in India.
The government has distributed 50 million Covaxin doses to the states but latest data showed the AstraZeneca shot appeared to be more popular in India.
As on Thursday, 16.3 million Indians had been inoculated.
With 11 million Covid-19 cases and 157,000 deaths, India is the world's second-worst affected country after the United States but it has posted a rapid decline in the number of cases in the past five months.
Export prospects
Bharat Biotech, based in the southern city of Hyderabad, signed a contract in February to deliver the vaccine to Brazil, and other countries like the Philippines and the United Arab Emirates are also interested, media reports said.
Business news website Moneycontrol said France, which has struggled to get its vaccination drive off the ground, may be interested in procuring vaccines from India, but Covaxin has not yet been validated by EU regulators.
French ambassador Emmanuel Lenain met Bharat Biotech's chief executive on Monday.
He said he was impressed by the company's commitment of making Covid-19 vaccine "a global public good, accessible and affordable throughout the world."
Last month, the French envoy had thanked India for supplying medicines to France during the pandemic.




 We’ll protect state wealth from opaque deals – Prof Jane Naana
We’ll protect state wealth from opaque deals – Prof Jane Naana
 Mauritania president says running for second term in June polls
Mauritania president says running for second term in June polls
 I won't ever say I was a mere driver’s mate' — Prof. Opoku-Agyemang
I won't ever say I was a mere driver’s mate' — Prof. Opoku-Agyemang
 2024 polls: 'EC struggling to defend credibility'— Prof. Opoku-Agyemang
2024 polls: 'EC struggling to defend credibility'— Prof. Opoku-Agyemang
 Akufo-Addo gov't's 'greed, unbridled arrogance, unrestrained impunity, sheer dis...
Akufo-Addo gov't's 'greed, unbridled arrogance, unrestrained impunity, sheer dis...
 Election 2024: Ghana needs an urgent reset, a leadership that is inspiring – Ma...
Election 2024: Ghana needs an urgent reset, a leadership that is inspiring – Ma...
 Partner NDC to rollout a future of limitless prospects – Prof Jane Naana Opoku-A...
Partner NDC to rollout a future of limitless prospects – Prof Jane Naana Opoku-A...
 NPP will remain in gov’t till Jesus comes — Diana Asamoah
NPP will remain in gov’t till Jesus comes — Diana Asamoah
 Sunyani Technical University demands apology from former SRC president over sex-...
Sunyani Technical University demands apology from former SRC president over sex-...
 'Dumsor' was resolved by Mahama but ‘incompetent' Akufo-Addo has destroyed the g...
'Dumsor' was resolved by Mahama but ‘incompetent' Akufo-Addo has destroyed the g...
