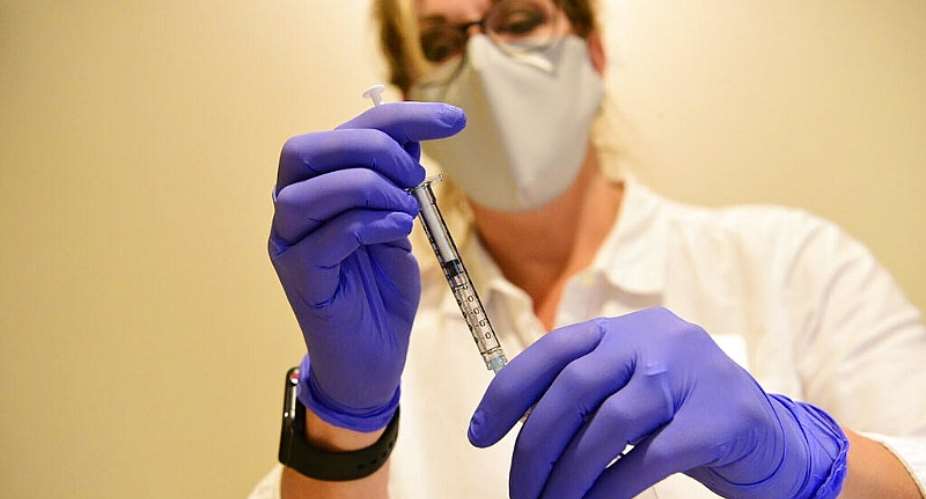
The European Union is likely to approve Johnson & Johnson's single-dose Covid-19 vaccine in early March, a French minister has said, after the United States cleared the shot for emergency use on Saturday.
The single-shot vaccine is highly effective in preventing severe Covid-19, including against newer variants, the Food and Drug Administration (FDA) said before giving it the green light.
French Industry Minister Agnes Pannier-Runacher told France 3 television that the European Medicines Agency (EMA) was also evaluating information transmitted by the US pharmaceuticals company.
An EU approval in early March would allow the vaccine to be rolled out in late March or early April, she said, adding this was "good news" because it offers protection with a single shot in contrast to other vaccines requiring two.
She said it was possible that a booster shot was needed later "but we can't be sure yet".
The EU hoped to receive 600 million doses of the vaccine by the end of June, she said.
France has so far vaccinated 1.5 million people, the minister said. By June all 15 million people belonging to the country's most vulnerable population segments will have been vaccinated, she added, acknowledging however that reaching that target might require a faster vaccine rollout.
In large clinical trials, the J&J vaccine's efficacy against severe disease was 85.9 percent in the United States, 81.7 percent in South Africa, and 87.6 percent in Brazil.
The J&J shot appears less protective than Pfizer and Moderna's two-shot regimens, which both have an efficacy of around 95 percent against all forms of Covid-19 from the classic coronavirus strain.
(AFP)




 Adu Boahen’s murder: Police arrest house help who was in possession of deceased’...
Adu Boahen’s murder: Police arrest house help who was in possession of deceased’...
 Akufo-Addo nominates Felicia Attipoe as Tema West MCE
Akufo-Addo nominates Felicia Attipoe as Tema West MCE
 Election 2024: I can't have someone I defeated twice as my successor – Akufo-Add...
Election 2024: I can't have someone I defeated twice as my successor – Akufo-Add...
 We’ll protect state wealth from opaque deals – Prof Jane Naana
We’ll protect state wealth from opaque deals – Prof Jane Naana
 Mauritania president says running for second term in June polls
Mauritania president says running for second term in June polls
 I won't ever say I was a mere driver’s mate' — Prof. Opoku-Agyemang
I won't ever say I was a mere driver’s mate' — Prof. Opoku-Agyemang
 2024 polls: 'EC struggling to defend credibility'— Prof. Opoku-Agyemang
2024 polls: 'EC struggling to defend credibility'— Prof. Opoku-Agyemang
 NPP will remain in gov’t till Jesus comes — Diana Asamoah
NPP will remain in gov’t till Jesus comes — Diana Asamoah
 Sunyani Technical University demands apology from former SRC president over sex-...
Sunyani Technical University demands apology from former SRC president over sex-...
 'Dumsor' was resolved by Mahama but ‘incompetent' Akufo-Addo has destroyed the g...
'Dumsor' was resolved by Mahama but ‘incompetent' Akufo-Addo has destroyed the g...
