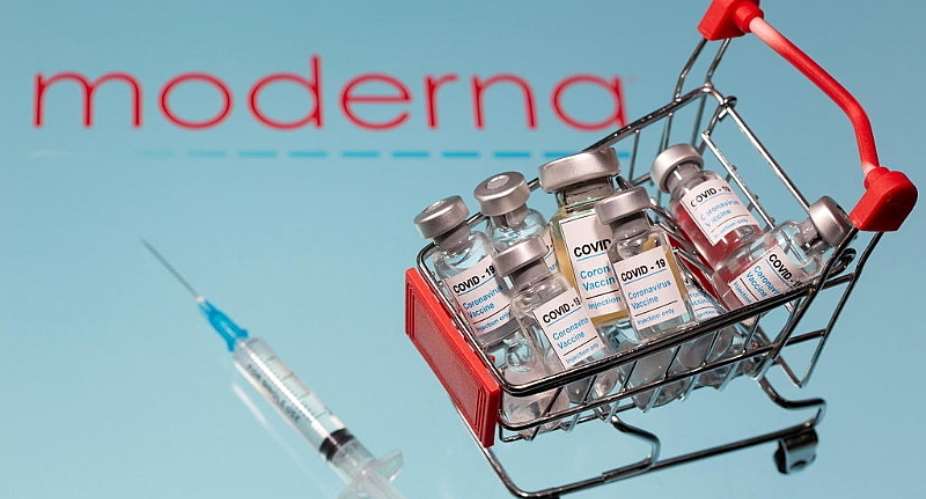
The US drugmaker Moderna has said it will apply for US and European emergency authorisation of its Covid-19 vaccine after a late-stage study showed it to be more than 94 percent effective.
“I allowed myself to cry for the first time,” Moderna's chief medical officer, Dr Tal Zaks, said of the vaccine, which also reported a 100 percent success rate in preventing severe Covid cases, with no major safety concerns.
“In the trial, we have already saved lives. Just imagine the impact then multiplied to the people who can get this vaccine.”
The news from Moderna comes less than two weeks after the Pfizer-BioNTech partnership announced its own vaccine candidate was 95 percent effective and also asked for fast-tracked approval.
US Health Secretary Alex Azar said the US Food and Drug Administration was expected to meet on 10 December to consider approving the Pfizer-BioNTech vaccine, with Moderna's to follow one week later.
The vaccines' progress offers hope to millions in the US and Europe, where a surge in second wave coronavirus infections has put hospitals under renewed strain.
- One in ten French admit escaping to their holiday homes during second lockdown
- Major Paris mosques stay closed due to 30-person limit under eased lockdown
France's health authority has said it wants any Covid-19 vaccination to be optional, in recommendations drawn up for the government.
Instead, it's hoped that the vast majority of French will be persuaded to get the jab by information campaigns.
Trump advisor quits
In separate news, a controversial member of US President Donald Trump's coronavirus task force has resigned from his post.
Scott Atlas, who questioned the need for masks and lockdown measures during his four months as a special advisor, stepped down on Monday.
In a tweet, Atlas said he "always relied on the latest science and evidence without any political consideration or influence".




 Dumsor: Don't rush to demand timetable; the problem may be temporary — Atik Moha...
Dumsor: Don't rush to demand timetable; the problem may be temporary — Atik Moha...
 Space X Starlink’s satellite broadband approved in Ghana — NCA
Space X Starlink’s satellite broadband approved in Ghana — NCA
 2024 election will be decided on the grounds of the economy; choice of running m...
2024 election will be decided on the grounds of the economy; choice of running m...
 Dumsor: We're demanding less; just give us a timetable — Kwesi Pratt to ECG
Dumsor: We're demanding less; just give us a timetable — Kwesi Pratt to ECG
 Do I have to apologise for doing my security work, I won’t – Simon Osei-Mensah r...
Do I have to apologise for doing my security work, I won’t – Simon Osei-Mensah r...
 All my businesses have collapsed under Akufo-Addo — NDC Central regional chair
All my businesses have collapsed under Akufo-Addo — NDC Central regional chair
 Military, Prison Officers clash in Bawku, three injured
Military, Prison Officers clash in Bawku, three injured
 GRA-SML contract: MFWA files RTI request demanding KPMG report
GRA-SML contract: MFWA files RTI request demanding KPMG report
 Court threatens to call second accused to testify if NDC's Ofosu Ampofo fails to...
Court threatens to call second accused to testify if NDC's Ofosu Ampofo fails to...
 Family accuses hospital of medical negligence, extortion in death of 17-year-old...
Family accuses hospital of medical negligence, extortion in death of 17-year-old...
