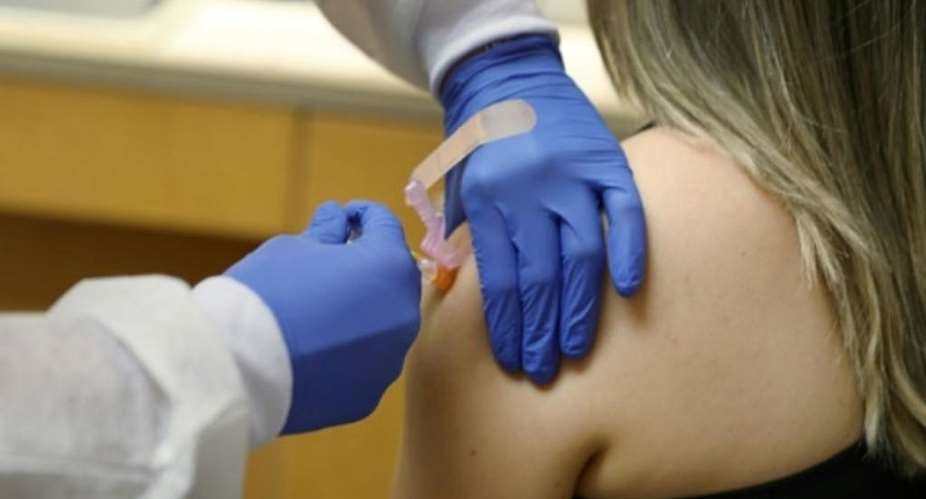
A promising coronavirus vaccine trial being carried out by pharma giant AstraZeneca and Oxford University has been suspended after a participant developed an “unexplained illness”.
AstraZeneca described the move as a "routine" pause, stressing the adverse reaction was only recorded in a single participant during Phase 3 tests that were being carried out in the UK, US, Brazil and South Africa.
Reports say the volunteer who fell ill is located in the UK and is expected to recover, although exact details of the adverse reaction suffered were not made public.
While some 180 potential vaccines against Covid-19 are being developed around the world, it's been hoped this vaccine would be among the first to come on the market – perhaps as early as January.
Following successful phase 1 and 2 testing, some 30,000 participants are involved in the late-stage trials before they were "voluntarily paused" to allow a review by an independent committee, AstraZeneca said.
"As part of the ongoing randomised, controlled global trials of the Oxford coronavirus vaccine, our standard review process was triggered and we voluntarily paused vaccination to allow review of safety data by an independent committee," a spokesperson told The Guardian newspaper.
Analysis by the BBC says this marks the second time the vaccine trial has been paused since the first lot of volunteers were immunised in April.
- France and three other EU countries sign deal for AstraZeneca Covid-19 vaccines
- Russia releases first batch of anti-Covid vaccine Sputnik V to the public
Given Phase 3 vaccine trials can last several years, the WHO has said it does not expect any Covid-19 vaccine to fully meet its efficacy and safety guidelines in order to be approved this year.
France is among four European countries that had signed deals to supply doses of the AstraZeneca-Oxford University vaccine, while on Monday Australia announced it would manufacture nearly 34 million doses should it prove effective.
Meanwhile Russia this week began rolling out its coronavirus vaccine, Sputnik V, to the general public as part of a “year-long mass vaccination campaign”, after a recent study showed the jab caused no adverse side-effects.




 Burkina Faso expels French diplomats for 'subversive activities'
Burkina Faso expels French diplomats for 'subversive activities'
 GOIL reduces petrol price by 29 pesewas, sells GHC14.70 per litre
GOIL reduces petrol price by 29 pesewas, sells GHC14.70 per litre
 The disrespect towards security is terrible; we can do better — Atik Mohammed co...
The disrespect towards security is terrible; we can do better — Atik Mohammed co...
 Starlink to cease connection in Ghana, other “unavailable” countries on April 30...
Starlink to cease connection in Ghana, other “unavailable” countries on April 30...
 MMCEs, DCEs and Regional Ministers must be elected to reduce political interfere...
MMCEs, DCEs and Regional Ministers must be elected to reduce political interfere...
 National Cathedral: ‘Nonsense; you take taxes from broke Ghanaians to dig a clum...
National Cathedral: ‘Nonsense; you take taxes from broke Ghanaians to dig a clum...
 April 18: Cedi sells at GHS13.59 to $1, GHS13.01 on BoG interbank
April 18: Cedi sells at GHS13.59 to $1, GHS13.01 on BoG interbank
 We must harness the collective power and ingenuity of female leaders to propel o...
We must harness the collective power and ingenuity of female leaders to propel o...
 Saglemi Housing Project will not be left to rot – Kojo Oppong Nkrumah
Saglemi Housing Project will not be left to rot – Kojo Oppong Nkrumah
 Asantehene commends Matthew Opoku Prempeh for conceiving GENSER Kumasi Pipeline ...
Asantehene commends Matthew Opoku Prempeh for conceiving GENSER Kumasi Pipeline ...
